Day 2 :
Keynote Forum
Massimo Guarnieri
University of Padua, Italy
Keynote: A selective hybrid stochastic approach to proton exchange membrane fuel cells multi parameter identification
Time : 09:00-09:40

Biography:
Massimo Guarnieri completed his MS degree in Electrical Engineering at University of Padua, Italy, in 1979 and his PhD degree in Electrical Science in Rome in 1987. He joined Italian National Council of Research in 1982 and University of Padua in 1983, where he has been Full Professor of Electrical Engineering since 2000. Initially, he worked on devices for thermonuclear fusion research. He later centered his research interests on electromagnetic computation. In the last ten years, he has been involved in modeling and designing electrochemical storage devices. He is interested in the history of technology and science. He is a Columnist and a member of the Editorial Board of IEEE Industrial Electronics Magazine.
Abstract:
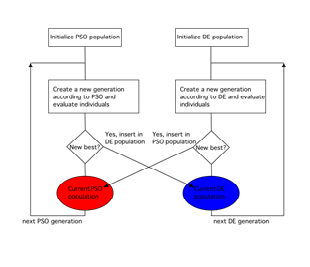
The characterization of fuel cells involves a number of physical parameters which are important for quantifying and comparing the performance of the materials and for trimming analytical and numerical models. Careful ex situ measurements of such parameters can be performed by means of a number of diagnostic techniques, whose results are however not completely consistent with fuel cell operation due to several side effect. Conversely, in situ measurements can provide meaningful operational values, but a very few techniques are available to determine a limited number of parameters. An alternative consists in multiple parameter identification from multiple fundamental measurements performed in different conditions, e.g., at different temperature, pressure, concentration, and humidification. If there is only one unknown parameter, the solution is easy, requiring just a statistical interpolating technique. In the case of multiple unknown parameters, the problem becomes increasingly challenging with the number of parameters, as duplicity problem emerges, i.e., several groups of parameters may lead to the same performance (e.g. polarization curve). A number of numerical tools have been proposed to face this kind of problems. Stochastic mathematical models have been applied to the analysis of fuel cells for more than ten years, but typically to specific problems and by means of semi-empirical models, with an increased number of articles published in the last years. We present an original formulation that makes use of an accurate zero-dimensional multi-physical model of a polymer electrolyte membrane fuel cell and of two cooperating stochastic algorithms, particle swarm optimization (PSO) and differential evolution (DE), that proved to be successful in extracting multiple material parameters (exchange current density, mass transfer coefficient, diffusivity, conductivity, and activation barriers) from the experimental data of multiple polarization curves (i.e., in situ measurements) under controlled temperature, gas back pressure and humidification. The method is suitable for application in other fields where fitting of multiphysics nonlinear models is involved.
Recent Publications:
1. Alotto P., Guarnieri M., Moro F., “Redox Flow Batteries for the storage of renewable energy: a review”, Renewable & Sustainable Energy Reviews 29 (2014): 325-335.
2. Guarnieri M., Alotto P., Moro F., “Modeling the Performance of Hydrogen-Oxygen Unitized Regenerative Proton Exchange Membrane Fuel Cells for Energy Storage”, Journal of Power Sources 297, (11), (2015): 23-32.
3. Guarnieri M., Negro E., Di Noto V., Alotto P., “A Selective Hybrid Stochastic Strategy for Fuel-Cell Multi-Parameter Identification”, Journal of Power Sources 332 (2016): 249–264.
4. Spagnuolo G., Petrone G., Mattavelli P., Guarnieri M., “Vanadium Redox Flow Batteries: Potentials and Challenges of an Emerging Storage Technology”, IEEE Industrial Electronics Magazine 10 (4), (2016): 20-31.
5. Maggiolo D., Picano F., Guarnieri M., “Flow and dispersion in anisotropic porous media: a Lattice-Boltzmann study”, Physics of Fluids, 28 (10), (2016): 102001.
6. Moro F., Trovò A., Bortolin S., Del Col D., Guarnieri M., “An alternative low-loss stack architecture for vanadium redox flow battery: comparative assessment”, Journal of Power Sources, 340 (2017)
Keynote Forum
Arunkumar Jayakumar
Auckland University of Technology, New Zealand
Keynote: Measurement techniques and related challenges involved in the gas diffusion electrode characterization of PEM fuel cell stack
Time : 09:40-10:20

Biography:
Arunkumar Jayakumar is a Research Fellow at Auckland University of Technology, New Zealand. He has 10 years of experience in PEM fuel cell stacks and systems. He has worked with wide range of Ballard’s stack namely, Nexa, 1020 ACS and 1310 WCS. His research activities include PEM fuel cell stacks and systems, sensor, electric vehicles, material characterization and hydrogen energy. His research is currently funded by the IBTec, AUT. He is a member of the IPENZ, IEEE and ASME.
Abstract:
Proton Exchange Membrane (PEM) fuel cells are emerging as a commercially viable alternative for the production of clean and reliable energy. The Membrane Electrode Assembly (MEA) is the principal component of a PEM fuel cell. The operation of the fuel cell involves the hydrogen (fuel) being supplied to the anode and oxygen/air being fed to the cathode. At the anode region, the hydrogen is oxidized to protons and electrons the membrane allows protons to pass through it while the electrons are forced to travel through the external circuit. At the cathode, the oxidant is reduced and in this way, electricity is drawn from the cell. Figure 1 illustrates a single PEM cell, indicating various sub-components and the charge transfer. PEM fuel cell performance is meticulously correlated to the gas diffusion electrodes (GDE). GDE in a PEM fuel cell stack usually comprises of the catalyst layer and the gas diffusion layer and the present paper provides a comprehensive measurement issues pertaining to the characterization of GDE. The various GDE characterizations (in situ and ex situ) and the corresponding instrument involved in the present paper is listed as follows in the table 1. Gas diffusion electrode characterization is complex, which involves the existence of both solid and fluid phases, and due to the random morphology of the diffusion electrode. However, these characteristics are very much significant to validate its role in the PEM fuel cell stack. The measuring instrument play a significant role in maximizing the efficiency and durability of the PEM fuel cell stack components; because it is impossible to control the operating parameters without proper measurement. However, the measurement strategies involved in the GDE components is highly complex due to the non-linear behaviour during the PEM fuel cell operation. In the present paper, a holistic insight on all these measurement instruments and related challenges will be comprehensively dealt.
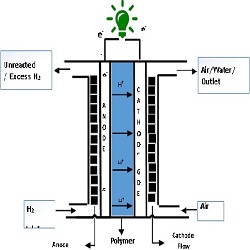
Figure 1: A plan of PEMFC single cell, indicating electron and ion transfer
Recent Publications:
1. Fernández, J.L., et al., Optimization Of “Wired” Enzyme O2â€Electroreduction Catalyst Compositions by Scanning Electrochemical Microscopy. Angewandte Chemie International Edition, 2004. 43(46): p. 6355-6357.
2. Yuhashi, N., et al., Development of a novel glucose enzyme fuel cell system employing protein engineered PQQ glucose dehydrogenase. Biosensors and bioelectronics, 2005. 20(10): p. 2145-2150.
3. Cosnier, S., A. Le Goff, and M. Holzinger, Towards glucose biofuel cells implanted in human body for powering artificial organs. Electrochemistry Communications, 2014. 38: p. 19-23.
- Primary Cell Batteries| Secondary Cell Batteries| Design and Technology of Batteries| Applications of Batteries| Latest Developments in Batteries
Location: Olimpica 2

Chair
Manfred Martin
RWTH Aachen University, Germany
Session Introduction
Taketoshi Matsumoto
Osaka University, Japan
Title: High capacity anode with Si nanopowder fabricated from swarf for lithium ion battery

Biography:
Taketoshi Matsumoto (Assoc. Prof., Sci, PhD) received his MSc and PhD degrees from Tokyo Institute of Technology, Yokohama, Japan in 2001. From 2000 to 2002, he was a Research Fellow of the Japan Society for the Promotion of Science. Since 2001, he was a Postdoctoral Research Associate in University of Southern California, CA, US. Since 2003, he was a Lecturer in University of Tsukuba, Tsukuba, Japan. Since 2004, he was a Research Associate in Institute for Molecular Science, Okazaki, Japan. Since 2007 and 2014, he has been an Assistant and Associate Professor, respectively, in the Institute of Scientific and Industrial Research, Osaka University, Osaka, Japan. He is interested in energy related nano-materials and devices such as TFTs, LSI, luminous materials, sealed permanent memories, solar cells and Li ion batteries.
Abstract:
Si swarf is generated during slicing Si ingots to produce Si wafers for solar cells. The weight of Si swarf, i.e., industrial waste, is nearly the same as that of Si wafers. Si swarf and Si nanopowder produced by the ball and beads milling methods possesses flake-like shape with length smaller than a few μm and thickness thinner than 40 nm. We have applied Si nanopowder produced from swarf to Li ion batteries, fluorescent materials, a hydrogen generation material, and solar cells. In this study, Si has been applied to high capacity active materials for anodes in Li ion batteries. Si nanoparticles smaller than 150 nm are known to show good cyclability; while for larger Si particles, peeling-off of Si due to their volume change occurs during lithiation and delithiation, resulting in degradation of the cyclability. Si swarf is a promising low-cost material for mass production of Si anodes, while Si nanoparticles have been fabricated so far using high-cost processes such as CVD and laser ablation. Cyclability of a Si anode fabricated from Si swarf is improved by addition of 10-15 wt% fluoroethylene carbonate (FEC) to ethylene carbonate (EC)/diethylene carbonate (DEC)=1/1 electrolyte solutions with 1 M LiPF6. The solutions form a thin and stable solid-electrolyte interphase (SEI) layer, leading to decreases in the SEI resistance (RSEI) and charge transfer resistance (Rct). Carbon-coating (C-coating) on Si also improves the cyclability of the Si anode. Limitation of the delithiation capacity at 1500 mA h/g after deep lithiation at 0.01 V with a Li foil counter electrode also shows better cyclability than that for limitation of the lithiation capacity at 1500 mA h/g after deep delithiation at 1.5 V.
Recent Publications:
1. Matsumoto T, Kimura K, Nishihara H, Kasukabe T, Kyotani T, Kobayashi H (2017) Fabrication of Si nanopowder from Si swarf and application to high-capacity and low cost Li-ion batteries. J. Alloys Compd. 720: 529-540.
2. Kimura K, Matsumoto T, Nishihara H, Kasukabe T, Kyotani T, Kobayashi H (2017) Improvement of cyclability of Li-ion batteries using C-coated Si nanopowder electrode fabricated from Si swarf with limitation of delithiation capacity. J. Electrochem. Soc. 164: A995-A1001.
3. Kasukabe T, Nishihara H, Kimura K, Matsumoto T, Kobayashi H, Kyotani T (2017) Beads-milling of waste Si sawdust into high-performance nanoflakes for lithium-ion batteries. Sci. Rep. 7: 42734-1-10.
4. Matsumoto T, Maeda M, Kobayashi H (2016) Photoluminescence enhancement of adsorbed species on Si. Nanoscale Res. Lett. 11: 7-1-6.
5. Matsumoto T, Maeda M, Furukawa J, Kim W-B, Kobayashi H (2014) Si nanoparticles fabricated from Si swarf by photochemical method. J. Nanopart. Res. 16: 2240-1-7.
6. Maeda M, Imamura K, Matsumoto T, Kobayashi H (2014) Fabrication of Si swarf and application to solar cells. Appl. Surf. Sci. 312: 39-42.
Jun-Hyung Ryu
Dongguk University, South Korea
Title: The development of a decision-making framework for intelligent energy storage system

Biography:
Jun-Hyung Ryu has expertise in Mathematical Programming in the Energy Systems. He has been mainly approached the energy system in the process industry with high energy consumption and concerned with developing the methodology to address multiple energy sources such as renewable energy sources and fossil fuels, and energy storage system. He is an Associate Professor in Department of Nuclear & Energy System Engineering at Dongguk University.
Abstract:
Statement of the Problem: Energy has become an important issue for process industry. In order to survive under tough competition, energy should be secured for the industry in terms of cost and amount. However, continuously varying energy conditions makes it unprecedentedly challenging. Any approach to mitigate the negative impact of the external and internal variation should be developed and implemented. Recently, Energy Storage System (ESS) has been emerging as a competitive tool. There is much to be done to take the full advantage of ESS in practice. This paper aims to highlight the key issues for the practical implementation.
Methodology & Theoretical Orientation: At first, rigorous decision-making framework should be developed for the operation of ESS. Many reports only mention the potential advantages of ESS without describing specific technologies and methodologies. The framework should be closed incorporated with energy system management. The forecasting of supply and demand should be made. The accurate and reliable forecasting allows the ESS to operate in robust manner. Energy and power saving from the perspective of suppliers can be different. The benefit of ESS should be rigorously evaluated by computing the profit. The profit by using ESS should be computed against the ESS set up cost during the entire economic lifespan. ESS is under development. New technologies are constantly developed and tested.
Findings: This presentation highlights the need to urgently develop decision-making frameworks for the systematic operation of ESS. This can be applied to any type of ESS.
Conclusion & Significance: ESS itself cannot generate energy nor consume it. The impact of ESS can be realized when a proper match between suppliers and demands is made. The rising energy and power cost and increasing waste due to the mismatch indicates the importance of ESS in energy systems. More works are expected to take full advantage of ESS.
Recent Publications:
1. Ryu J H, Lee S B, Hodge B M, Lee I B (2016) Techno-economic simulation approach in preparation of employing renewable energies for process industry. IEEE Photovoltaic Specialists Conference 1862-1864
2. Ryu J H, Hodge B M (2016) Mathematical Modelling-based Energy System Operation Strategy considering Energy Storage Systems. Computer Aided Chemical Engineering 38:1455-1460
3. Lee S, Ryu J H, Hodge B M, Lee I B (2016) Development of a Neural Network-based Renewable Energy Forecasting Framework for Process Industries, Computer Aided Chemical Engineering 38: 1527-1532
4. Han J H, Ryu J H, Lee I B(2013), Multi-objective optimization design of hydrogen infrastructures simultaneously considering economic cost, safety and CO2 emission, Chemical Engineering Research and Design 91(8):1427-1439
5. Ryu J H (2013) Developing an integrated capacity planning framework for production processes and demand supply chains, Korean Journal of Chemical Engineering 30: 27-32
Chunman Zheng
National University of Defense Technology, China
Title: Structural Change and Thermal Properties of NCA/LiMn2O4 Blend Materials during the Charging Process

Biography:
Abstract:
Layered oxides LiNi0.8Co0.15Al0.05O2 (NCA) is one of the most attractive cathode material for high power applications such as hybrid/plug-in hybrid electrical vehicles. It shows great benefits in high volumetric energy density and high rate performance. However, there are still some technical hurdles to be solved, such as high temperature stability. The poor high temperature stability is attributed to the chemical reaction of NCA cathode material with the electrolyte in the process of charging and discharging. Blending with other stable cathode materials is one effective way to improve the properties of materials. For example, Tran et al. reported the blend material of LiMn2O4 and NCA, demonstrating very stable cycle performance and high temperature stability. In the present work, NCA/LiMn2O4 blend materials with different ratio were prepared in order to improve the properties of materials. Structural change and thermal properties of NCA/LiMn2O4 blend materials during the charging process were systematically studied. Figure 1 shows the XRD patterns of NCA/LiMn2O4 blend materials which were charged to 4.2 V and 5.0 V, respectively. It shows that the layered cathode materials undergo a reversible phase transition, namely a phase transition from H1 phase to M phase (3.748 V), M phase to H2 phase (4.017 V), and H2 phase to H3 phase (4.227 V). During the phase transition, the cell parameters of blended materials a and b decreases whereas c increases. Figure 2 shows the temperature change of NCA/LiMn2O4 blend materials during the charging process and the high temperature stability of blend materials which were charged to 4.2 V. When the mass ratio of LiMn2O4 is 20%, the blend cathode materials exhibit better anti-overcharge behavior with less heat generated. The decomposition temperature of the blend materials which were charged to 4.2 V is higher than the pure NCA. The blend materials with 20% LiMn2O4 have the best temperature stability.
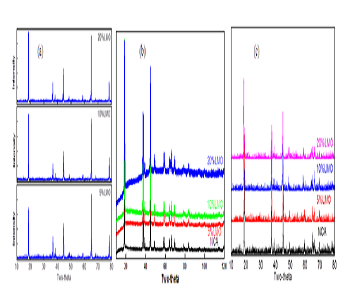
Figure 1: XRD patterns of (a) NCA/LiMn2O4 blend materials, (b) blend materials charged to 4.2V, (c) blend materials charged to 5.0V.
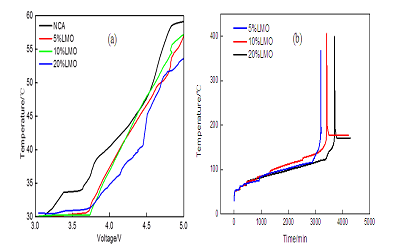
Figure 2: (a) The temperature change of NCA/LiMn2O4 blend materials during the charging process, (b) high temperature stability of blend materials charged to 4.2V.
Recent Publications:
1. Li X, Xie Z, Liu W, Ge W, Wang H, and Qu M. Effects of fluorine doping on structure, surface chemistry, and electrochemical performance of LiNi0.8Co0.15Al0.05O2 [J]. Electrochimica Acta, 2015, 174: 1122-1130.
2. Haselrieder W, Ivanov S, Tran H Y, Theil S, Froböse L. Influence of Formulation Method and Related Processes on Structural, Electrical and Electrochemical properties of LMS/NCA-blend Electrodes [J]. Progress in Solid State Chemistry, 2014, 42(4): 157-174.
3. Tran H Y, Täubert C, Fleischhammer M, Axmann P, Küppers L and Mehrens M W. LiMn2O4 Spinel/ LiNi0.8Co0.15Al0.05O2 Blends as Cathode Materials for Lithium-ion batteries [J]. Journal of the Electrochemical Society, 2011, 158(5): A556-A561.
Katsutoshi Ono
Kyoto University, Japan
Title: Theories of on-board hydrogen redox electric power generators for infinite cruising range electric vehicles

Biography:
Katsutoshi Ono received B. Eng. Degree from Kyoto University, Japan, in 1961, degree of Dr. Sci. from Faculté des Sciences, Université de Paris in 1967. He was researcher at Ecole des Mines de Paris, 1965-1967, Professor of Materials Science, Kyoto University, 1982-1997, Energy Science & Technology, 1997-2001. He is Currently Professor Emeritus.
Abstract:
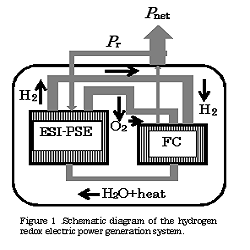
This paper presents the theoretical aspects of a specific propulsion system for electric vehicles, based on a prototype standard passenger fuel cell vehicle (FCV), termed a hydrogen redox electric power generator (HREG). This generator utilizes a combined energy cycle composed of a fuel cell that produces power and an electrostatic- induction potential superposed water electrolytic cell (ESI-PSE) for the synthesis of a stoichiometric H2/O2 fuel for the fuel cell. To allow an essentially infinite cruising range, the fuel must be synthesized while the car is in motion. In this scenario, the HREG offers considerable advantages. Firstly, this device is capable of functioning with zero matter and energy inputs and generates no emissions, without violating the laws of thermodynamics. The H2O → H2 +1/2O2 reduction reaction proceeds in the ESI-PSE, which functions on a so-called “zero power input” mechanism involving the conversion of electrostatic energy to chemical energy. In this unit, the power used is 17% of the total electrical energy that is theoretically required, while the remaining 83% can be provided by electrostatic energy free of power. Part of the power delivered by the fuel cell is returned to the ESI-PSE cell, while the remainder represents the net power output used to drive the electric motor. A second advantage is that the HREG system can be employed on both a large scale, such as in the case of a central power station, and on a much smaller scale, such as for on-board electric power generation in an electric vehicle. The present work performed a theoretical assessment of this new propulsion system for electric vehicles, focusing on the following three aspects:
1. the power electronics circuit connected to the ESI-PSE cell,
2. the performance of the on-board HREG system,
3. the lithium-ion battery charge-discharge reciprocating electric power generator.
Recent Publications:
1. Ono K: (2015) Energetically self-sustaining electric power generation system based on the combined cycle of electrostatic induction hydrogen electrolyzer and fuel cell IEEJ, Trans. on Fundamentals and Materials, Vol.135 No.1 pp. 22-33.
2. Ono K: (2016) Hydrogen redox electric power and hydrogen energy generators, International Journal of Hydrogen Energy, vol.41 PP.10284-102913.
3. Ono K: (2016) “Hydrogen redox electric power and hydrogen energy generators”, International Journal of Hydrogen Energy, vol.41 P.10284-10291
4. Barbir F, PEM fuel cells, Theory and Prracice, Elsevier, Amsterdam P.268..
5. Annual Report, National Resources and Energies 1999/2000, The Agency for Resources and Energies, Japan.
6. O’ Hayre R, Cha S.W, Collela W and Prinz F.B., (2006) Fuel cell, Fundamentals, John Wiley & Sons INC.
Shridhar Pandey
Ramway New Energy Co. Ltd., China
Title: Primary lithium batteries passivation characteristics and effects in LiSOCl2 chemistries
Time : 14:50-15:15

Biography:
Shridhar Pandey is working as a Country Head at Ramway New Energy Co. Ltd., China. He has completed his graduation in Electronics and Communications Engineering at Visvesvaraya Technological University, Belgaum. Being an adaptable and innovative Engineer with over three years of experience in Manufacturing and Marketing, he has excellent team leading capabilities. He has been working since a year and focusing on Lithium Battery Technology for AMI/AMR, Smart Meters and Energy Meters. He has introduced Ramway batteries to utility companies and Indian market. He has also been successful in writing articles and research papers based on batteries and AMI for various Indian magazines and journals.
Abstract:
Passivation is a phenomenon for lithium battery; a thin chemical film appears on the surface of lithium anode and prevents it from oxidation which is forming on the surface of the metal. In lithium-thionyl chloride battery, thionyl chloride is a liquid. Lithium anode gets in touch with thionyl chloride and the oxidation reaction will start slowly. The outcome of this oxidation is lithium chloride. The lithium chloride which is formed on the surface of the lithium anode is very small and it prevents the chemical reaction between lithium and thionyl chloride. This phenomenon of lithium is called as passivation. The passivation in lithium thionyl chloride batteries starts as soon as the batteries are manufactured, but the reaction is not fast. The passivation is directly proportional to the temperature. With the increase in temperature, the rate of passivation increases. The passivation is more dangerous as longer the time is. Passivation is the intrinsic characteristics of lithium thionyl chloride battery. It is impossible to store lithium thionyl chloride batteries without passivation. The lithium chloride is formed on the surface of the lithium anode in thionyl chloride is very dense which prevents the reaction between lithium and thionyl chloride. Because of passivation the self-discharge rate inside the cell becomes very small. By this way, we can achieve the shelf life of 10 years for lithium thionyl chloride batteries. This is the positive sign of passivation. Passivation protects the battery capacity and prevents it from capacity loss. In this presentation, we are going to deliver how to save batteries from getting passivized and how it can be depassivized.
Recent Publications:
1. Shridhar Pandey, Anish Garg(2017) Two Pole Latching relays for Smart Electricity Meters at Metering India 2017
2. Shridhar pandey, Anish Garg(2016) Performance, testing and Reliability of Two Pole Latching relays for smart Energy meters at World energy Congress 2016, Italy
3. Shridhar pandey, Anish Garg(2016) Performance, testing and Reliability of Two Pole Latching relays for smart Energy meters Renewable energy week, London UK 2016
4. Shridhar pandey, Anish Garg(2016) Performance, testing and Reliability of Two Pole Latching relays for smart Energy meters at Water and Energy International Journal, New Delhi
Yujie Li
National University of Defense Technology, China
Title: Nano-sized lithium-rich cathode 0.4Li2 MnO3 0.6Li[Ni1/3Mn1/3Co1/3]O2 prepared by resorcinol-formaldehyde assisted sol-gel method

Biography:
Yujie Li studies energy materials and their electrochemistry, including synthesis of cathode material, anode material, and all-solid lithium battery as well as their applications in lithium storage and conversion. He received his Bachelor’s degree in Materials Chemistry at Lanzhou University, China in 2003; Master’s degree in Materials Physics Chemistry in 2006 and; Doctor’s degree in Materials Science & Engineering in 2010 at National University of Defense Technology, China. He is now an Assistant Professor in Department of Materials Science & Engineering at National University of Defense Technology, China.
Abstract:
Recently, layered lithium-rich cathode materials, xLi2MnO3 (1-x)LiMO2(M=transition metal) have been considered as one of the most promising cathode materials due to their high specific capacity (200-300 mAhg-1) and high operating voltage. However, lithium-rich cathode materials still suffer from several major defects, such as low coulomb efficiency, the poor long-term capacity retention, and the inferior rate performance. As we know, nano-sized cathode is an effective method, the nano dimension provided more active surface sites for lithium storage, and also shortened the lithium-diffusion pathways, and these could improve electrochemistry performance. Herein, we prepared nano particle lithium-rich layered 0.4Li2MnO30.6Li[Ni1/3Mn1/3Co1/3]O2 by resorcinol-formaldehyde assisted sol-gel method. Nano-sized lithium-rich cathode presented excellent cycling performance. It showed a high initial discharge capacity of 256.8mAh g-1 and an initial coulomb efficiency of 78.9%. The discharge capacity after 50 cycles was 240.2mAh g-1 with capacity retention of 93.5% due to more active surface sites and shortens lithium-diffusion pathways of nanoparticles. Figure 1(a)(b) demonstrate its SEM images. Lithium-rich nanoparticles are about 250 nm-300 nm. Figure 1(c) showed the XRD pattern, it formed the good layered crystallizability. Figure 1(d) demonstrates, it has good capacity retention. These results indicate the nano-sized lithium-rich cathode could effectively suppress capacity attenuation and enhance coulomb efficiency and cycling performance.
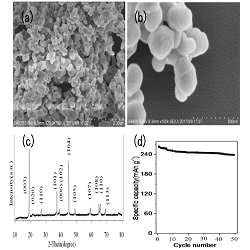
Figure 1: (a) (b) SEM, (c) XRD and (d) cycling performance (0.1C rate) of 0.4Li2MnO3 0.6Li[Ni1/3Mn1/3Co1/3]O2 nanoparticles
Recent Publications:
1. Wang C-C, Jarvis K A, Ferreira P J, Manthiram A. Effect of Synthesis Conditions on the First Charge and Reversible Capacities of Lithium-Rich Layered Oxide Cathodes[J]. Chem. Mater., 2013, 25(15): 3267-3275.
2. Pramanik A, Ghanty C, Majumder S B. Synthesis and electrochemical characterization of xLi(Ni0.8Co0.15Mg0.05)O2· (1-x)Li[Li1/3Mn2/3]O2(0.0
Liu Shuangke
National University of Defense Technology, China
Title: Graphene oxide coated S particles with long cycle life for lithium sulfur battery

Biography:
Liu Shuangke studies energy materials and electrochemistry, including synthesis of metal oxides, nano-carbons, sulfur-carbon composites as well as their applications in lithium storage and conversion. He received his Bachelor’s degree in Materials Science & Engineering at Hunan University, China in 2010; Master’s degree in Applied Chemistry in 2012 and; Doctorate degree in Materials Science & Engineering in 2016 at National University of Defense Technology, China. He is now an Assistant Professor in Department of Materials Science & Engineering at National University of Defense Technology, China.
Abstract:
Lithium sulfur battery has been regarded as one of the most promising high energy density rechargeable energy storage system for next generation due to its high theoretical specific capacity/energy density, natural abundance and environmental friendliness. However, the rapid capacity fade during long cycles which is caused by polysulfide shuttle and volume expansion during cycles tremendously inhibits its practical application. Building coated architecture of sulfur is one effective way to confine sulfur in the sulfur cathode thus enables stable cycle life. For example, Zhou et al. reported a sulfur/GO core-shell particle, in which sulfur particle were well wrapped by GO, demonstrating very stable cycle performance up to 1000 cycles. Herein, we also prepared GO-coated sulfur particles to enhance the cycle performance of lithium sulfur battery. Different from the above example which uses milled nano-sulfur particles, we use sodium sulfide and sodium sulfite to generate sulfur particles in aqueous GO solution followed by centrifugation and freeze-drying process, which results in in situ coating structure. The SEM (Fig. 1 a) and TEM (Fig. 1 b, c) images clearly demonstrate sulfur particles are well wrapped by wrinkled GO sheets, the high resolution TEM result indicate the crystal phase of sulfur particles. Due to the intact coating structure of GO and good chemical bond between sulfur and GO, the GO-coated sulfur particle cathode shows excellent cycling performance. Figure 1d demonstrates its cycle performance at 1 C rate, the initial discharge capacity is 513.4 mAhg-1, with 467.4 mAhg-1 retained after 400 cycles, corresponding to capacity retention of 91%. However, after 400 cycles, the capacity fades starts to speed up with 260.6 mAhg-1 and 225.1 mAhg-1 left after 800 and 1000 cycles. These results indicate that the physical GO coating could effectively suppress polysulfides shuttle effect and enhance the cycling performance but cannot completely eliminate it.
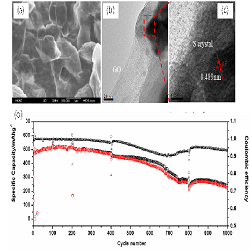
Figure 1: (a) SEM, (b) TEM, (c) high resolution TEM and (d) cycling performance at 1C rate of GO-coated S particles
Recent Publications:
1. Manthiram A, Fu Y, Chung S.H, Zu C, Su Y.S. Rechargeable Lithium-Sulfur Batteries, Chem. Rev. 2014, 114(23):11751-11787.
2. Wang D W, Zeng Q, Zhou G, Yin L, Li F, Cheng H M, Gentle I, Lu G. Carbon-sulfur composites for Li-S batteries: status and prospects. J. Mater. Chem. A, 2013, 1 (33): 9382~9394.
3. Rong J.P, Ge M.Y, Fang X, Zhou C.W. Solution Ionic Strength Engineering as a Generic Strategy to Coat Graphene Oxide (GO) on Various Functional Particles and Its Application in High-Performance Lithium-Sulfur (Li-S) Batteries, Nano Lett., 2014, 14,473-479.
Jungmyoung Kim
Changwon national University, South Korea
Title: An analysis of vanadium redox flow battery performance under various operating conditions

Biography:
Jungmyoung Kim earned his Master’s degree in Vanadium Redox Flow Battery Studies from Changwon National University in 2017. He is currently a PhD student at the Nano thermofluidics Energy Transfer Lab of Mechanical Engineering Department of Changwon National University in South Korea.
Abstract:
The vanadium redox flow battery (V-RFB) used in a large-capacity energy storage system is a semi-permanent secondary battery having a relatively long life span and low electrolyte contamination due to cross-over. The performance of the V-RFB system depends on conditions such as electrolyte flow rate and temperature, which are key operating variables. This paper devises four reservoir systems with a single cell 25 cm2 reaction areas and temperature, flow rate control to understand the general thermal and electrochemical reaction characteristics of V-RFB. Also, experimental analysis of a single cell is presented by measuring polarization curves according to experimental variables. The polarization curves are measured in the low current density region without the influence of the concentration overpotential, and the electrolyte temperatures are 278 K, 298 K, and 318 K. the current density of the constant current discharge from 50 A/m2 to 300 A/m2 and the flow rates are 20 mL/min, 60 mL/min, and 100 mL/min. In the polarization curve analysis, the influence of the activation overpotential according to the experimental conditions is applied to the Tafel theory, and the influence of the concentration loss is ignored. The electron transfer coefficient increases as the electrolyte temperature and flow rate increase, while the exchange current density is obtained as described in the Arrhenius equation. The overpotential due to the resistance is represented by the area specific resistance and decreases to the ration of 20.3 (mΩ cm2)/K as the temperature of the electrolyte increases. In the analysis, we successfully found the electrochemical parameters and resistances of the overpotential by verifying various temperature and flow rate.
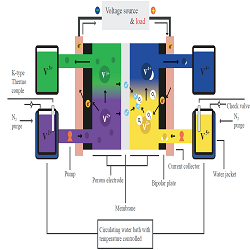
Figure 1: Single cell schematic with temperature control system and four electrolyte reservoirs.
Recent Publications:
1. Ulaganathan M, et al. (2016) Recent advancements in all-vanadium redox flow batteries. Advanced materials interfaces 3.1.
2. Rose M A, Williamson M A, Willit J (2015) Determining the exchange current density and Tafel constant for uranium in LiCl/KCl eutectic. ECS electrochemistry letters 4.1:C5-C7.
3. Xiao S, et al. (2016) Broad temperature adaptability of vanadium redox flow battery—Part 1: Electrolyte research. Electrochimica acta 187:525-534.
4. Zhang C, et al. (2015) Effects of operating temperature on the performance of vanadium redox flow batteries. Applied energy 155:349-353.
5. Mohamed M R, Leung P K, Sulaiman M H (2015) Performance characterization of a vanadium redox flow battery at different operating parameters under a standardized test-bed system. Applied energy 137:402-412
Ashley Brew
OXIS Energy Ltd, UK
Title: Microgrid energy storage using Lithium-Sulfur Batteries: Feasibility of solvent-in-salt electrolytes

Biography:
Ashley Brew gained his MSc in Catalysis and PhD in Electrocatalysis from Cardiff University, specializing in the oxygen reduction reaction in fuel cells. After a few post-doctoral positions in the fields of EPR spectroscopy and thermoelectric materials, he now works at OXIS Energy, a leading developer of lithium-sulfur battery chemistry. As a research scientist at OXIS, Ashley is a member of the electrolyte research group and is currently nearing the end of a 12-month project that aims to establish the feasibility of novel electrolytes for microgrid applications. OXIS Energy also develop lithium-sulfur secondary batteries for several other applications, including automotive and aerospace.
Abstract:
Lithium-Sulfur (Li-S) batteries are considered one of the most promising technologies that could provide a generational leap in terms of energy density over current lithium ion batteries. OXIS Energy have demonstrated this by succeeding in developing Li-S batteries at 400Wh/kg. However, capacity fade at such high energy density is rapid and further research and development is needed to alleviate this. Many factors contribute to capacity fade in Li-S batteries: for example, dissolution and loss of cathode material, consumption of the electrolyte due to its reaction with lithium metal and electrical isolation of insulating sulfur and Li2S charge and discharge products. Another major issue in Li-S batteries is the ‘polysulfide shuttle’, in which reaction intermediates shuttle between the cathode and anode during charge. So-called solvent-in-salt (SIS) electrolytes are those in which the salt exceeds the solvent either by weight, by volume, or both. These unique electrolytes have demonstrated interesting properties in the literature and may solve many of the problems outlined above. SIS electrolytes inhibit intermediate dissolution due to the common ion effect, thus reducing active material loss and inhibiting the polysulfide shuttle. SIS electrolytes have also demonstrated improved lithium plating due to the high lithium-ion transference number, leading to lower rates of electrolyte depletion. These combinations of factors have resulted in these electrolytes exhibiting excellent cycle stability and coulombic efficiency in literature studies. Here we will present our work developing this type of electrolyte for R&D pouch cells and their possible use in microgrid energy storage applications.
Recent Publications:
1. M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescub and G. J. Offer, Energy Environ. Sci., 2015, 8, 3477-3494.
2. T. Zhang, M. Marinescu, L. O'Neill, M. Wild and G. Offer Phys. Chem. Chem. Phys., 2015, 17, 22581-22586.
3. A. Fotouh, D. J. Auger, K. Propp, S. Longo, M. Wild, Renewable and Sustainable Energy Reviews, 2016, 56, 1008-1021.
4. K. Propp, M. Marinescu, D. J. Auger, L. O'Neill, A. Fotouh, K. Somasundaram, G. Offer, G. Minton, Journal of Power Sources, 2016, 328, 289-299
5. G.Minton, L. Lue, Journal of Molecular Physics, 2016, 114, 16-17, 2477-2491
- Classification of Fuel Cells| Applications of Fuel Cells| Hydrogen Energy| Super Capacitors vs. Battery| Various Energy Materials
Location: Olimpica 2

Chair
Arunkumar Jayakumar
Auckland University of Technology, New Zealand
Session Introduction
Nai-Chien Shih
Ming Dao University, Taiwan
Title: Development of a pure oxygen and pure hydrogen fuel cell generator for small under water vehicles

Biography:
Nai-Chein Shih completed his PhD from University of Cranfi eld, UK. He is now the Chairman of Chinese Academy of Science and Education, 2017 – Present. He
has his specializations in Hydrogen technology and fuel cell power generation system (PLC control and IC control), Business management and energy product
engineering and development.
Abstract:
This study has developed and tested a 1-KW water cooled Proton Exchange Membrane Fuel Cell Generator (PEMFCG) system. This system that provides 1 KW of electric power steadily at ~60°C has been tested under software monitory online via pure H2 and O2 recycling. The PEMFCG is equipped with two sets of gas-liquid dual-purpose compressors and H2/O2/H2O vapor separators. The water discharged from the separators is being used for continuous power generation. The PEMFCG will be disassembled and then reassembled in an Underwater Vehicle (UV) prototype for performance retest under actual operating environment.
Serge Zhuiykov
Ghent University Global Campus, South Korea
Title: Supercapacitor with improved performance based on ALD-developed two-dimensional WO3/TiO2 heterojunction

Biography:
Serge Zhuiykov received his PhD in Materials Science and Engineering in 1991. He has more than 25 years of combined academic and industrial experience working at different universities in Australia, Japan, Korea and Europe and industrial environments. Since 2015, he is a Senior Full Professor in Department of Applied Analytical & Physical Chemistry of Ghent University Global Campus, Korea and a Director of Environmental & Energy Research Center. His research interests include “The development, design and fabrication of new two-dimensional nanomaterials for solid-state environmental sensors and other advanced functional devices”. He has published more than 200 peer-reviewed scientific publications including two monographs in 2007 and 2014, respectively. He is a recipient of the 2007, 2011, 2013 Australian Academy of Science/Japan Society for Promotion of Science and 2010 Australian Government Endeavour Executive Awards for his work on Advanced Nanomaterials.
Abstract:
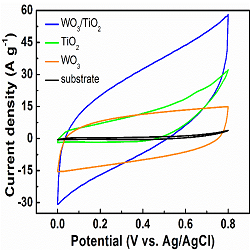
Supercapacitors (SCs) also known as, electrochemical capacitors, have been considered as one of the potential energy storage systems in addition to batteries, due to their high power density, fast charging/discharging and long-term cycling stability. Many metal oxide including RuO2, MnO2, Co3O4, NiO, SnO2, TiO2, WO3, perovskites, ferrites, etc., have been applied as alternative materials for supercapacitor electrodes. Among these metal oxides, TiO2 and WO3 are two of the most versatile materials and have been widely applied in electronics, photocatalysts, sensors and electrochromism. In the past, we demonstrated high capabilities of monolayers of TiO2 and WO3 developed by atomic layer deposition (ALD). It is appeared to indicate that both of them are promising candidate materials for high-performance SC electrodes. Moreover, nanostructured WO3/TiO2 heterojunction was highly investigated in various applications, especially in photochemistry and photo-electrochemistry, and has already exhibited excellent electrochemical properties. However, atomically-thin two-dimensional (2D) WO3/TiO2 heterojunction have not yet been considered and investigated as the electrode material in SCs. Therefore, in this study, we demonstrate for the first time capabilities of the 2D WO3/TiO2 heterojunction developed by ALD technique for SC electrodes. CV curves of three 2D nanomaterials at scan rate of 50 mV s-1 are presented in figure below. The ALD-developed WO3/TiO2 heterojunction has a uniform thickness of ~12 nm with a relative rough surface. The SC electrode based on this heterojunction has shown excellent super-capacitive behavior with high specific capacitance up to 625.53 F g-1 at the current density of 1 A g-1 and remarkable long-term stability of 97.98 % over 2000 cycles.
Recent Publications:
1. Lockhande C D et al (2011) Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 11:255-270.
2. Bavykin D V et al (2006) Protonated titanates and TiO2 nanostructured materials: Synthesis, properties, and applications Adv. Mater. 18:2807-2824.
3. Zheng H et al (2011) Nanostructured tungsten oxide - Properties, synthesis, and applications. Adv. Funct. Mater. 21:2175-2196.
4. Zhuiykov S et al (2017) Wafer-scale fabrication of conformal atomic-layered TiO2 by atomic layer deposition using tetrakis (dimethylamino) titanium and H2O precursors. Mater. Des. 120:99-108.
5. Zhuiykov S et al (2017) Atomic layer deposition-enabled single layer of tungsten trioxide across a large area. Appl. Mater. Today 6:44-53.
Mamta Kumari
Homi Bhabha National Institute, India
Title: Fuel cell performance of SPEEK-PEG-PWA composite membrane

Biography:
Mamta Kumari is currently working as a Senior Research Fellow in the Membrane Development Section of Chemical Engineering Group at Homi Bhabha National Institute, India. She has expertise in the field of development of solid polymer electrolyte for electrochemical applications, e.g., fuel cells. Her studies are in the field of development of structurally modified membranes with high conductivity.
Abstract:
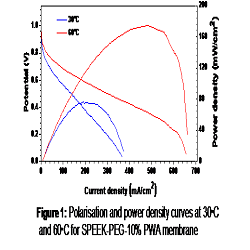
Proton exchange membrane fuel cell (PEMFC) is promising technology for clean and efficient power generation in the 21st century. Currently perfluorinated membranes are used as electrolyte that have high cost and complicated synthesis process which limits the commercialization of the PEMFC. Hydrocarbon polymers are good alternative for membranes and during last few decades research has been concentrated on that. The present work focuses on the development of composite membrane by using a thermoplastic polymer, partially sulfonated polyether ether ketone (SPEEK) and polyethylene glycol (PEG) as cross-linker along with varying amount of phosphotungstic acid (PWA) as an inorganic additive. The membranes are prepared using sulfonated PEEK with an ion exchange capacity of 2 meq/g and 33% of PEG having molecular weight of 600 Da was used as cross linker, the amount of PWA was varied from 5 to 50%. Among the various concentration of PWA in membranes, the 10 wt% PWA gave highest conductivity (90 mS/cm), less swelling and good stability in water up to 60ºC. The fuel cell performance of these membranes was measured using a 25 cm2 membrane electrode assembly made using commercial carbon paper based electrode (0.2 mg/cm2 Pt loading). The cell performance tests were carried out (H2/O2 at 2 bar pressure) at 30 and 60ºC (Figure 1). The maximum power density obtained at 30ºC was 75 mW/cm2 at current density of 200 mA/cm2 and voltage 375 mV which increased at 60ºC to 173 mW/cm2 at current density of 440 mA/cm2 and voltage 400 mV. The cell gave stable performance after running for 40 hours at its maximum power. The present membrane performance is comparable with the reported value for commercial membranes (power density of 360 mW/cm2 to 420 mW/cm2 at 600 mV and 80ºC). Hence, the SPEEK-PEG-PWA membranes can be an alternative solid polymer electrolyte for fuel cell.
Recent Publications:
1. Yee R S L, Rozendal R A, Zhang K, Ladewig B P (2012) Cost effective cation exchange membranes: A review. Chemical Engineering Research and Design 90:950-959.
2. Sambandam S, Ramani V (2007) SPEEK/functionalized silica composite membranes for polymer electrolyte fuel cells. Journal of Power Sources 170:259-267.
3. Moreno G N, Molina C M, Gervasio D, Robles P F J (2015) Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renewable and Sustainable Energy Reviews 52:897-906.
4. Lee H K, Chu Y J, Kim R A, Nahm S K, Kim J C, Yoo J D (2013) Densely sulfonated block copolymer composite membranes containing phosphotungstic acid for fuel cell membranes. Journal of Membrane Science 434:35-43.
5. Colicchio I, Wen F, Keul Helmut, Simon U, Moeller M (2009) Sulfonated poly(ether ether ketone)-silica membrane doped with phosphotungstic acid. Morphology and proton conductivity. Journal of Membrane Science 326:45-57
Mohamed A Abu-Saied
Advanced Technologies and New Materials Research Institute, Egypt
Title: Preparation and characterization of PVC-SP(ST-co-AN) membranes for proton exchange membrane fuel cell applications

Biography:
Mohamed A. Abu-Saied has his expertise in polyelectrolyte membranes for fuel cell Application. His open and contextual evaluation (preparation and characterization) of polymer polyelectrolyte membranes for fuel cell application. He has built this model after years of experience in research, evaluation, teaching and administration in education institutions.
Abstract:
Polyvinyl chloride-sulfonated poly(styrene-co-acrylonitrile) [PVC-SP(ST-co-AN)] for proton exchange membrane fuel cell have been prepared through two steps. The first step was preparation of sulfonated poly(styrene-co-acrylonitrile) [SP(ST-co-AN)] by using solution polymerization and KPS as initiator and sulfonated the product of poly(styrene-co-acrylonitrile) [P(ST-co-AN)] which reacts with sulfuric acid. The second step was mixing polyvinyl chloride (PVC) and sulfonated poly(styrene-co-acrylonitrile) [SP(ST-co-AN)] with different ratio. Essential characters required for polyelectrolyte fuel cell membrane especially ionic conductivity, methanol permeability, ion exchange capacity (IEC), thermal stability and high mechanical properties were investigated. Ion exchange capacity increase with increase sulfonated poly(styrene-co-acrylonitrile) [SP(ST-co-AN)]. The methanol permeability of membrane which consider as essential character for PEM fuel cell application was found lower than of nafion. The obtained results are very promising and opening new area for conducting further investigations considering the very low price of polyvinyl chloride compared to nafion.
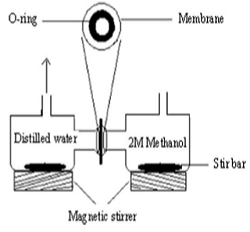
Figure 1: Glass diffusion cell for methanol permeability measurements.
Recent Publications:
1. Harper C (2009) The neuropathology of alcohol-related braindamage. Alcohol Alcohol 44:136-140.
2. Zhu Y, Chen H, He C (2011) J Polym Res 18:1409
3. Mohy Eldin MS, Elzatahry AA, El-Khatib KM, Hassan EA, ElSabbah MM, Abu-Saied MA (2011) J Appl Polym Sci 119:120
4. Abu-SaiedMA,ElzatahryAA,El-KhatibKM,HassanEA,El-Sabbah MM, Drioli E, Mohy Eldin MS (2012) J Appl Polym Sci 123:3710
5. Mohy Eldin MS, Abu-Saied MA, Elzatahry AA, El-Khatib KM, Hassan EA, El-Sabbah MM (2011) Int J Electrochem Sci 6:5417
6. Wycisk R, Trochimczuk WM (1991) J Appl Polym Sci 43:1727
7. Mohammadi T, Skylls-Kazacos M (1995) J Membr Sci 98:77
8. Elkady MF, Abu-Saied MA, Abdel Rahman AM, Soliman EA, ElzatahryAA,YossefME,MohyEldinMS(2011)Desalination279:152
9. Kim M, Saito K (1999) React Funct Polym 40:275
Arunkumar Jayakumar
Auckland University of Technology, New Zealand
Title: The potential of micro-fuel cells to power the pacemaker-A critical assessment on material selection

Biography:
Arunkumar Jayakumar is a Research Fellow at Auckland University of Technology, New Zealand. He has 10 years of experience in PEM fuel cell stacks and systems. He has worked with wide range of Ballard’s stack namely, Nexa, 1020 ACS and 1310 WCS. His research activities include PEM fuel cell stacks and systems, sensor, electric vehicles, material characterization and hydrogen energy. His research is currently funded by the IBTec, AUT. He is a member of the IPENZ, IEEE and ASME.
Abstract:
Fuel cells are one of the efficient energy systems which convert the chemical energy of the fuel directly into the electrical energy. The greatest advantage of a fuel cell is its potential to be used for wide range of applications starting from high power vehicles to low power Implantable medical devices (IMDs). IMDs can be classified into two categories namely functional assisted devices and in-vivo measurements devices. Among these two devices, the role of pacemakers is very crucial as they require reliable power. Conventional, pacemakers are powered by lithium-ion batteries that need to be replaced from time to time which result in the increase in cost and complexity as well severe inconvenience to the patients. A glucose fuel cell (GFC) which is a subset of conventional polymer electrolyte membrane fuel cells (using hydrogen or alcohols as fuels) that oxidizes glucose and reduces oxygen to give electric energy. Since, glucose and oxygen are both present and continuously replenished in physiological fluids by the metabolism, this revolutionary approach is theoretically able to provide enough energy along the patient lifetime without any need of battery replacements. However, appropriate material selection is a critical factor in the design of these kind of fuel cells. In the present paper a holistic assessment on various material considering various factors such as bio-compatibility, strength to weigh ratio and other desirable characteristics inline to pacemaker design benchmarks are studied.
Recent Publications:
1. Fernández, J.L., et al., Optimization Of “Wired” Enzyme O2â€Electroreduction Catalyst Compositions by Scanning Electrochemical Microscopy. Angewandte Chemie International Edition, 2004. 43(46): p. 6355-6357.
2. Yuhashi, N., et al., Development of a novel glucose enzyme fuel cell system employing protein engineered PQQ glucose dehydrogenase. Biosensors and bioelectronics, 2005. 20(10): p. 2145-2150.
Renato Pelosato
Politecnico di Milano, Italy
Title: Doping strategies in NdBaCo2 O5+δ layered perovskites cathodes for IT-SOFCs

Biography:
Renato Pelosato had his degree in Chemistry at the Pavia University in 1999, and PhD in Materials Engineering at Politecnico di Milano in 2006. Since 2001, he is working on advanced ceramic materials for application in solid oxide fuel cells. Particularly he is devoted to the synthesis of materials using different synthetic routes (solid state reactive firing, wet chemical methods). In his career, he acquired interest in and knowledge on the structural, microstructural and physico-chemical characterization of ceramic materials. He is author of more than 40 papers published in peer-reviewed International Journals.
Abstract:
Co-based layered perovskites (LnBaCo2O5+d Ln=Lanthanides or Y) have been recently proposed as cathode materials for IT-SOFCS. These compounds crystallize in a double perovskite structure (LnCoO3-δ–BaCoO3-δ), where Ln and Ba ions sit on alternated single perovskite layers. Pr, Nd and Gd based compounds show the best performance, likely due to their optimum ion size. Recently, chemical modifications like cation deficiency and Co substitution have gained interest as a way to tailor the defect chemistry of these materials. The effect of Fe-doping on the B-site and of Ba deficiency on the A-site has been investigated. XRD, TG-DTA, ICP-MS and cerimetric titrations were performed to assess the phase composition, crystal structure, chemical composition and oxygen content. The electrochemical properties were investigated via 4-probe electrical conductivity measurements and electrochemical impedance spectroscopy on symmetrical cells with GDC electrolyte. All the compounds show high total electrical conductivity (between 400 and 600 S/cm at 700°C). A tenfold decrease of the area specific resistance is observed at increasing Ba deficiency to 10%. Detailed equivalent circuit analysis reveals that the effect of Ba is associated with a promotion of the bulk diffusion steps at high frequency. On the other hand, iron substitution triggers a structural change from orthorhombic to tetragonal, and lowers the electrical conductivity. The compound with 20% of iron shows the lowest polarization resistance (0.17 Ω*cm2 at 700°C). The ORR mechanism investigated by ECM and physical 1D modeling shows that the first electronation of the oxygen atom on the MIEC surface and the bulk diffusion of the oxygen vacancies in the MIEC lattice are the rate determining steps.
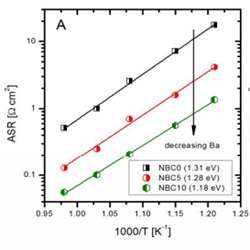
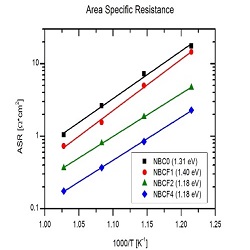
Figure 1: ASR of NBC material in symmetric cells with GDC electrolyte. Effect of increasing Ba deficiency (left) and increasing Iron doping (right)
Recent Publications:
1. A. Tarancón, S. J. Skinner, R. J. Chater, F. Hernández-Ramírez and J. A. Kilner, Journal of Materials Chemistry, 2007, 17, 3175. http://dx.doi.org/10.1039/b704320a
2. R. Pelosato, G. Cordaro, D. Stucchi, C. Cristiani and G. Dotelli, Journal of Power Sources, 2015, 298, 46. http://dx.doi.org/10.1016/j.jpowsour.2015.08.034
3. S. Pang, X. Jiang, X. Li, Q. Wang, Z. Su, J. Power Sources, 204 (2012) 53-59. http://dx.doi.org/10.1016/j.jpowsour.2012.01.034
4. F. Jin, H. Xu, W. Long, Y. Shen, T. He, J. Power Sources 2013, 243, 10. http://dx.doi.org/10.1016/j.jpowsour.2013.05.187
5. A. Donazzi, R. Pelosato, G. Cordaro, D. Stucchi, C. Cristiani, G. Dotelli, I.N. Sora, Electrochim. Acta, 182 (2015) 573-587. http://dx.doi.org/10.1016/j.electacta.2015.09.117
Giovanni Dotelli
Politecnico di Milano, Italy
Title: Graphene oxide membranes as alternative electrolytes for PEM fuel cells

Biography:
Giovanni Dotelli had his degree in Chemical Engineering at the Politecnico di Milano in 1989, and PhD in Materials Engineering at Politecnico di Milano in 1993. He is Associate Professor of Materials Science and Head of the Laboratory of Materials for Energy and Environment (Mat4En2) at the Dept. of Chemistry, Materials and Chemical Engineering “G.Natta” of the Politecnico di Milano. His research interests range from materials for fuel cells and environmental applications to sustainability assessment of industrial processes. He is author of more than 120 papers published in peer-reviewed international journals and conference proceedings.
Abstract:
Preliminary results about the use of a graphene oxide membrane as a possible alternative electrolyte to proton exchange membranes in Polymer Electrolyte Membrane Fuel Cells (PEMFCs) are reported. The key component of a PEMFC is the ionomer electrolyte, typically Nafion, due to its high proton conductivity in standard operating conditions, good mechanical properties and its effectiveness as gas separator. However, Nafion limits fuel cell operating temperature, reduces its conductivity upon dehydration and suffers shrinkage and swelling if water content changes significantly. An interesting alternative aiming to enhance FC efficiency and to overcome drawbacks related to Nafion employment could be represented by graphene oxide (GO). GO membranes have been found impermeable to many gases, whilst allowing permeation of water and minimizing fuel crossover. Some works report the use of GO in composite membranes with known proton conductors polymers such as Nafion, SPEEK, PBI and PVA. We synthesized and characterized a pure GO membrane as self-standing electrolytic membrane. A uniform self-assembled membrane was obtained and morphologically characterized by SEM, TGA and FT-IR analysis (Figure 1). A homogeneous surface and the presence of functional groups, i.e. hydroxyl, epoxy oxygen and carboxyl groups, which should guarantee proper proton conductivity, was achieved. A thickness of around 2 μm was measured. Since thickness is strongly related to initial GO solution concentration, other experiments are going to be done in order to increase it. Proton conductivity of the prepared membrane was determined by using impedance spectroscopy in the frequency range 1 MHz-1 Hz with an AC amplitude of 500 mV. A value of 0.008 S/cm was found at 20°C and anhydrous conditions. This is a very promising result since at the same operating conditions Nafion 212 shows a conductivity of almost one magnitude lower. Measurement at different temperatures and relative humidities, as well as in situ electrical tests, are in progress.
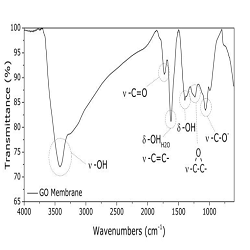
Figure 1: FT-IR spectrum of the GO self-standing membrane
Recent Publications:
1. Sansotera M, Gola M, Dotelli G, Navarrini W (2007). Chapter 7: The Role of Perfluoropolyethers in the Development of Polymeric Proton Exchange Membrane Fuel Cells. RSC Polymer Chemistry Series 24:158-178.
2. Li Q, He R, Jensen J O, Bjerrum N J (2003). Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C. Chem. Mater. 15:4896-4915.
3. Nair R R, Wu H A, Jayaram P N, Grigorieva I V, Geim A K (2012). Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 335:442-444.
4. Bayer T, Bishop S R, Nishihara M, Sasaki K, Lyth S M (2014). Characterization of a graphene oxide membrane fuel cell. J. Power Sources 272:239-247.
5. Changyan X, Xiaomei S, An J, Lina S, Chen Z, Yunqi C (2015). Fabrication and Characteristics of Reduced Graphene Oxide Produced with Different Green Reductants. Plos One 10 12:1-15.
Yuan-Lung Hsu
National Taiwan University, Taiwan
Title: Development of a Bi-Cell proton exchange membrane fuel cell with optimized groovedesigned piezoelectric actuator

Biography:
Yuan-Lung Hsu received his BS and MS degree from National Kaohsiung University of Applied Sciences in 2010 and 2013, respectively. He is currently a PhD candidate in the Department of Mechanical Engineering, National Taiwan University, Taiwan. His research focuses on the development of piezoelectric actuators application in PEMFC.
Abstract:
This study develops an air-breathing pump driven by a piezoelectric actuator to provide oxygen for a proton exchange membrane fuel cell (PEMFC) stack. A groove-designed PZT actuator enclosed with poly-di-methyl-siloxane (PDMS) can reduce uneven air feeding. This actuator can also improve the performance of both sides of a bi-cell, with only a 0.7% difference in the open-circuit voltage under the PZT actuator within a PDMS diaphragm (PZT-PDMS combination) with 30 min curing time, one side of the single cell had an open-circuit voltage of 0.989 V. We combined the permanent magnets with piezoelectric actuator. As the result, the three air-breathing pumps of the bi-cell stack are driven by only one piezoelectric actuator through the magnetic force between magnets. The design of the bi-cell stack enables the air-breathing pump to provide sufficient oxygen for both single cells simultaneously. According to the analysis of computational fluid dynamics, when the nozzle and diffuser of the air-breathing pump have aspect ratio 13.13, diffuser angle (θ) of 15°, and channel opening width (D) of 1.0 mm, the air flow uniformly distribute over inside of the pump, and then it helps stable reaction between oxygen and membrane electrode assembly (MEA). This aspect ratio is chosen to apply on the stack. When both the PZTmag and the PDMSmag used a magnet with a 6 mm diameter and 1 mm thinness, the maximum amplitude of 87 μm was produced under operating conditions of 70 Hz and 40 Vrms, and the power consumption was 0.03 W. The resulting maximum net power density of the bi-cell PZTmag–PEMFC stack was 0.1925 W cm-2, the proposed PEMFC has a net power density that is 20% more than that of a three bi-cell PZT-PEMFC reported in previous research (Ma, 2013), with 68% and 76% less volume and weight, respectively.
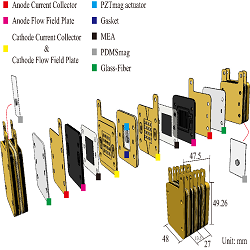
Figure 1: The schematic of the Bi-cell PZTmag-PEMFC stack.
Recent Publication:
1. D. Fuard, T. Tzvetkova-Chevolleau, S. Decossas, P. Tracqui, P. Schiavone (2008) Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectronic Engineering 85:1289-1293.
2. A. Ullmann (1998) The piezoelectric valve-less pump—performance enhancement analysis. Sens. Actuators A: Phys. 69:97-105.
3. H.K. Ma, S.H. Huang, B.R. Chen, L.W. Cheng, J (2008) Numerical study of a novel micro-diaphragm flow channel with piezoelectric device for proton exchange membrane fuel cells. Power Sources 180:402-409.
4. H.K. Ma, S.H. Huang, J.S. Wang, C.G. Hou, C.C. Yu, B.R. Chen, J (2010) Experimental study of a novel piezoelectric proton exchange membrane fuel cell with nozzle and diffuser. Power Sources 195:1393-1400.
5. H.K. Ma, H.M. Cheng, W.Y. Cheng, F.M. Fang, W.F. Luo, J (2013) Development of a piezoelectric proton exchange membrane fuel cell stack (PZT-Stack). Power Sources 240:314-322.
Po-Ching Hsu
National Taiwan University, Taiwan
Title: Magnetically driven piezoelectric proton exchange membrane fuel cell stack with built-in manifold

Biography:
Po-Ching Hsu is a MS student from Department of Mechanical Engineering at National Taiwan University. He has specialization in Thermodynamics and Fluid Dynamics. He is pursuing his research on piezoelectric proton exchange membrane fuel cell stack.
Abstract:
In this study, a magnetically driven piezoelectric proton exchange membrane fuel cell stack with built-in manifold is developed. To optimize the piezoelectric actuator (PZT actuator), several PDMS diaphragms with different Young’s moduli are fabricated and integrated with the PZT operating under voltage and frequency ranges of 10–50 V and 15–300 Hz, respectively. The effect of the sizes of magnets used (with the PZT actuator) on the performance of the PZTmag and the PDMSmag is investigated. On the other hand, to simplify the hydrogen pipeline and reduce the total volume and weight of the fuel cell stack, manifolds are developed to uniformly provide hydrogen to each cell using only one pipeline. As a result, the PZT actuator (with a curing time of 30 min for the PDMS) produces the largest amplitude of 1602 μm under the resonance frequency of 135 Hz and voltage of 40 V. The power consumption is 0.073 W. This optimized PZT actuator is used in the air breathing pumps of the magnetically driven PEMFC stack. When both the PZTmag and the PDMSmag use magnets 6 mm in diameter, a maximum amplitude of 87 μm is produced under operating conditions of 70 Hz and 40 V, which can provide sufficient air to the stack. The power consumption is 0.03 W.
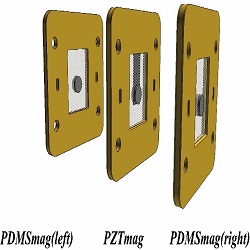
Figure 1: The Schematic of the air breathing pump components of magnetically driven PEMFC stack
Recent Publications:
1. D. Fuard, T. Tzvetkova-Chevolleau, S. Decossas, P. Tracqui, P. Schiavone (2008) Optimization of poly-di-methyl-siloxane (PDMS) substrates for studying cellular adhesion and motility. Microelectronic Engineering 85:1289-1293.
2. A. Ullmann (1998) The piezoelectric valve-less pump—performance enhancement analysis. Sens. Actuators A: Phys. 69:97-105.
3. H.K. Ma, S.H. Huang, B.R. Chen, L.W. Cheng, J (2008) Numerical study of a novel micro-diaphragm flow channel with piezoelectric device for proton exchange membrane fuel cells. Power Sources 180:402-409.
4. H.K. Ma, J.S. Wang, Y.T. Chang, J (2011) Development of a novel pseudo bipolar piezoelectric proton exchange membrane fuel cell with nozzle and diffuser. Power Sources 196:3766-3772.
5. H.K. Ma, H.M. Cheng, W.Y. Cheng, F.M. Fang, W.F. Luo, J (2013) Development of a piezoelectric proton exchange membrane fuel cell stack (PZT-Stack). Power Sources 240:314-322.

















