
Giovanni Dotelli
Politecnico di Milano, Italy
Title: Graphene oxide membranes as alternative electrolytes for PEM fuel cells
Biography
Biography: Giovanni Dotelli
Abstract
Preliminary results about the use of a graphene oxide membrane as a possible alternative electrolyte to proton exchange membranes in Polymer Electrolyte Membrane Fuel Cells (PEMFCs) are reported. The key component of a PEMFC is the ionomer electrolyte, typically Nafion, due to its high proton conductivity in standard operating conditions, good mechanical properties and its effectiveness as gas separator. However, Nafion limits fuel cell operating temperature, reduces its conductivity upon dehydration and suffers shrinkage and swelling if water content changes significantly. An interesting alternative aiming to enhance FC efficiency and to overcome drawbacks related to Nafion employment could be represented by graphene oxide (GO). GO membranes have been found impermeable to many gases, whilst allowing permeation of water and minimizing fuel crossover. Some works report the use of GO in composite membranes with known proton conductors polymers such as Nafion, SPEEK, PBI and PVA. We synthesized and characterized a pure GO membrane as self-standing electrolytic membrane. A uniform self-assembled membrane was obtained and morphologically characterized by SEM, TGA and FT-IR analysis (Figure 1). A homogeneous surface and the presence of functional groups, i.e. hydroxyl, epoxy oxygen and carboxyl groups, which should guarantee proper proton conductivity, was achieved. A thickness of around 2 μm was measured. Since thickness is strongly related to initial GO solution concentration, other experiments are going to be done in order to increase it. Proton conductivity of the prepared membrane was determined by using impedance spectroscopy in the frequency range 1 MHz-1 Hz with an AC amplitude of 500 mV. A value of 0.008 S/cm was found at 20°C and anhydrous conditions. This is a very promising result since at the same operating conditions Nafion 212 shows a conductivity of almost one magnitude lower. Measurement at different temperatures and relative humidities, as well as in situ electrical tests, are in progress.
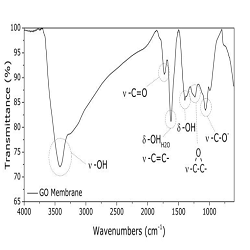
Figure 1: FT-IR spectrum of the GO self-standing membrane
Recent Publications:
1. Sansotera M, Gola M, Dotelli G, Navarrini W (2007). Chapter 7: The Role of Perfluoropolyethers in the Development of Polymeric Proton Exchange Membrane Fuel Cells. RSC Polymer Chemistry Series 24:158-178.
2. Li Q, He R, Jensen J O, Bjerrum N J (2003). Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C. Chem. Mater. 15:4896-4915.
3. Nair R R, Wu H A, Jayaram P N, Grigorieva I V, Geim A K (2012). Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 335:442-444.
4. Bayer T, Bishop S R, Nishihara M, Sasaki K, Lyth S M (2014). Characterization of a graphene oxide membrane fuel cell. J. Power Sources 272:239-247.
5. Changyan X, Xiaomei S, An J, Lina S, Chen Z, Yunqi C (2015). Fabrication and Characteristics of Reduced Graphene Oxide Produced with Different Green Reductants. Plos One 10 12:1-15.

