
Chunman Zheng
National University of Defense Technology, China
Title: Structural Change and Thermal Properties of NCA/LiMn2O4 Blend Materials during the Charging Process
Biography
Biography: Chunman Zheng
Abstract
Layered oxides LiNi0.8Co0.15Al0.05O2 (NCA) is one of the most attractive cathode material for high power applications such as hybrid/plug-in hybrid electrical vehicles. It shows great benefits in high volumetric energy density and high rate performance. However, there are still some technical hurdles to be solved, such as high temperature stability. The poor high temperature stability is attributed to the chemical reaction of NCA cathode material with the electrolyte in the process of charging and discharging. Blending with other stable cathode materials is one effective way to improve the properties of materials. For example, Tran et al. reported the blend material of LiMn2O4 and NCA, demonstrating very stable cycle performance and high temperature stability. In the present work, NCA/LiMn2O4 blend materials with different ratio were prepared in order to improve the properties of materials. Structural change and thermal properties of NCA/LiMn2O4 blend materials during the charging process were systematically studied. Figure 1 shows the XRD patterns of NCA/LiMn2O4 blend materials which were charged to 4.2 V and 5.0 V, respectively. It shows that the layered cathode materials undergo a reversible phase transition, namely a phase transition from H1 phase to M phase (3.748 V), M phase to H2 phase (4.017 V), and H2 phase to H3 phase (4.227 V). During the phase transition, the cell parameters of blended materials a and b decreases whereas c increases. Figure 2 shows the temperature change of NCA/LiMn2O4 blend materials during the charging process and the high temperature stability of blend materials which were charged to 4.2 V. When the mass ratio of LiMn2O4 is 20%, the blend cathode materials exhibit better anti-overcharge behavior with less heat generated. The decomposition temperature of the blend materials which were charged to 4.2 V is higher than the pure NCA. The blend materials with 20% LiMn2O4 have the best temperature stability.
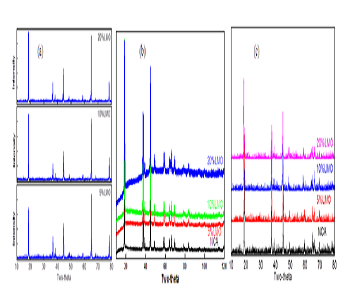
Figure 1: XRD patterns of (a) NCA/LiMn2O4 blend materials, (b) blend materials charged to 4.2V, (c) blend materials charged to 5.0V.
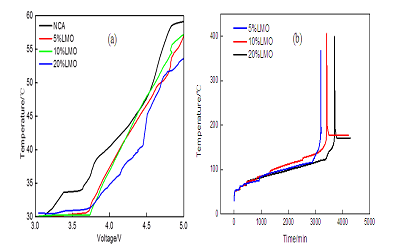
Figure 2: (a) The temperature change of NCA/LiMn2O4 blend materials during the charging process, (b) high temperature stability of blend materials charged to 4.2V.
Recent Publications:
1. Li X, Xie Z, Liu W, Ge W, Wang H, and Qu M. Effects of fluorine doping on structure, surface chemistry, and electrochemical performance of LiNi0.8Co0.15Al0.05O2 [J]. Electrochimica Acta, 2015, 174: 1122-1130.
2. Haselrieder W, Ivanov S, Tran H Y, Theil S, Froböse L. Influence of Formulation Method and Related Processes on Structural, Electrical and Electrochemical properties of LMS/NCA-blend Electrodes [J]. Progress in Solid State Chemistry, 2014, 42(4): 157-174.
3. Tran H Y, Täubert C, Fleischhammer M, Axmann P, Küppers L and Mehrens M W. LiMn2O4 Spinel/ LiNi0.8Co0.15Al0.05O2 Blends as Cathode Materials for Lithium-ion batteries [J]. Journal of the Electrochemical Society, 2011, 158(5): A556-A561.

