Day 1 :
Keynote Forum
Manfred Martin
RWTH Aachen University, Germany
Keynote: Oxygen ion conducting materials for energy conversion in fuel cells and batteries
Time : 09:30-10:10

Biography:
Manfred Martin is Professor and Head of the Institute of Physical Chemistry of RWTH Aachen University, Germany. At Seoul National University, Korea he was WCU Professor and is now Adjunct Professor. He has more than 30 years of experience in education and research of physical chemistry of solids as well as service at department, faculty and university level. His current research focusses on materials for energy conversion, resistive switching, solid-state reactions, secondary ion mass spectrometry, and computer simulations as well. Professor Manfred Martin has published >200 scientific papers in international, refereed journals. He received the Carl-Wagner Award and has been elected as member of the Royal Society of Chemistry. He has supervised more than 50 Ph.D. students and more than 20 postdoctoral fellows.
Abstract:
Interest in materials exhibiting oxygen ion conduction has increased owing to their great importance for energy conversion in Solid Oxide Fuel Cells (SOFC), Solid Oxide Electrolyser Cells (SOEC) and Rechargeable Oxide Batteries (ROB). Ceria-based oxides are regarded as key oxide materials because rare earth-doped ceria shows high oxygen ion conductivity even at intermediate temperatures. Using Density-Functional Theory (DFT), we have investigated defect formation and migration energies as well. Using Kinetic Monte Carlo (KMC) simulations, we then investigated the oxygen ion conductivity. We show that all interactions between the defects, namely vacancy-dopant attraction, dopant-dopant repulsion and vacancy-vacancy repulsion contribute to the so-called conductivity maximum of the ionic conductivity. Solid oxide electrolyser cells based on yttria-doped zirconia as electrolyte were operated for 6100 h and 9000 h, respectively. They were analyzed concerning degradation by various electron microscopy as well as micro-analytical techniques. We found several degradation phenomena such as formation of nano-sized pores at grain boundaries, formation of SrZrO3 at the interface electrolyte/anode and agglomeration of nickel particles in the cathode. The origin of these degradation phenomena is discussed in terms of the mass transport processes in the electrolyte caused by the two applied driving forces, namely the electrical potential and the oxygen potential gradient. Finally the new concept of Rechargeable Oxide Batteries (ROB) will be discussed.
Recent Publications:
1. Grope B, Zacherle T, Nakajama M, Martin M (2012) Oxygen ion conductivity of doped ceria: a Kinetic Monte Carlo study. Solid State Ionics 225:476-483.
2. Grieshammer S, Grope B, Koettgen J, Martin M (2014) A combined DFT + U and Monte Carlo study on rare earth doped ceria, Phys. Chem. Chem. Phys. 16:9974-9986.
3. The D, Grieshammer S, Schroeder M, Martin M, Al Daroukh M, Tietz F, Schefold J, Brisse A (2015) Microstructural comparison of solid oxide electrolyser cells operated for 6100 h and 9000 h. Journal of Power Sources 27: 901-911.
4. Grieshammer S, Nakayama M, Martin M (2016) Association of defects in doped non-stoichiometric ceria from first principles. Phys. Chem. Chem. Phys. 18:3804-3811.
5. Koettgen J, Zacherle T, Grieshammer S, Martin M (2017) Ab initio calculation of the attempt frequency of oxygen diffusion in pure and samarium doped ceria, Phys. Chem. Chem. Phys. DOI: 10.1039/c6cp04802a
Keynote Forum
Muhammad Afzal
KTH Royal Institute of Technology, Sweden
Keynote: Semiconductor-ionic materials for low temperature solid oxide fuel cells and electrolyte-layer free fuel cells
Time : 10:10-10:50

Biography:
Muhammad Afzal completed his MSc in Applied Physics at KTH Royal Institute of Technology, Stockholm, Sweden in 2013 and is pursuing PhD in Energy Technology at KTH Royal Institute of Technology, Stockholm, Sweden since June, 2014. After MSc at KTH University, he was appointed as Research Engineer and Project Manager at KTH for EC FP7 TriSOFC, STEM and Swedish Research Council (VR) projects. Currently, he is an emerging well known scientist in Solid Oxide Fuel Cell and Electrolyte-layer Free Fuel Cell (EFFC) and Manager for Advanced Fuel Cell and Solar Cell Group at KTH. His current work focuses on Semiconductor-ionic materials (three in one) for the development of EFFC technology working at low temperatures (300-600°C). He is an International Referee for International Journal of Hydrogen Energy, J. Phys Chem B & C, J. Scanning, Electrochimica Acta, and Advanced Energy Materials. He is Guest Editor of International Journal of Scanning. He has published more than 20 papers in refereed international journals.
Abstract:
In this work, demonstration of new advanced materials for advanced solid oxide fuel cells (ASOFCs) is reported to lower the operating temperature of solid oxide fuel cells (SOFCs). Nanocomposite semiconductor-ionic ceramic materials are prepared by solid state route through ball milling process and investigated as the catalytic electrode for low temperature solid oxide fuel cells (LTSOFCs) as type I device and core material for electrolyte-layer free fuel cell technology as type II device illustrated in Fig. 1. Synthesized perovskite oxides have exhibited great electrical conductivities, especially the Ba0.5Sr0.5Co0.8Fe0.2O3-δ prepared by co-precipitation method has shown a maximum conductivity up to 313 S/cm in air at 550°C measured by DC 4 probe technique. Similarly, Ni0.8Co0.15Al0.05Li (NCAL) oxide has shown balance electrical and ionic conductivity which is very useful for fuel cell performance. Additional advantages of BSCF and NCAL with both ionic and electronic conductivities are their cost effectiveness and low working temperature below 600°C. XRD analysis on the powdered form of BSCF sample exhibited the phase structure as perovskite oxide. Microstructure studies of the samples have revealed homogeneous structure and morphology of the nanoparticles using scanning electron microscopy (SEM). The prepared materials including semiconductor-ionic and perovskite materials have shown very good mechanical strength and stability proving their importance in advanced fuel cell technology using spark plasma sintering technique. Power densities for new energy conversion technology using our synthesized materials are measured between 600-1000 mWcm-2.
Image
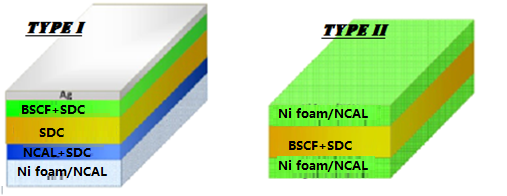
Figure 1: Schematic for SOFC (Type I) and EFFC (Type II).
Recent Publications:
1. Bin Zhu*, Peter Lund, Rizwan Raza, Ying Ma, Liangdong Fan, Muhammad Afzal et al, Schottky junction effect on high performance fuel cells based on nanocomposite materials, accepted by Adv. Energy Mater. 1401895 (2015) 1-6
2. Huiqing Hu, Qizhao Lin, Zhigang Zhu, Xiangrong Liu, Muhammad Afzal, Yunjuan He, Bin Zhu, Effects of Composition on the Electrochemical Property and Cell Performance of Single Layer Fuel Cell, J. Power Sources 275 (2015) 476-482
3. Huiqing Hu, Qizhao Lin, Afzal Muhammad, Bin Zhu, Electrochemical study of lithiated transition metal oxide composite for single layer fuel cell, J. Power Sources 286 (2015) 388-393
4. Muhammad Afzal, Rizwan Raza, Shangfeng Du, Raquel Bohn Lima, Bin Zhu, Synthesis of Ba0.3Ca0.7Co0.8Fe0.2O3-δ composite material as novel catalytic cathode for ceria-carbonate electrolyte fuel cells, Electrochimica Acta, Volume 178, 1 October 2015, Pages 385-391
5. Bin Zhu, Yizhong Huang, Liangdong Fan, Ying Ma, Baoyuan Wang, Chen Xia, Muhammad Afzal, Bowei Zhang, Wenjing Dong, Hao Wang, Peter D. Lund, Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle, Nano Energy, Volume 19, January 2016, Pages 156-164
6. Yunjuan He, Liangdong Fan, Muhammad Afzal, Manish Singh, Wei Zhang, Yufeng Zhao, Junjiao Li, Bin Zhu, Cobalt oxides coated commercial Ba0.5Sr0.5Co0.8Fe0.2O3−δ as high performance cathode for low-temperature SOFCs, Electrochimica Acta, Volume 191, 10 February 2016, Pages 223-229
7. Wenjing Dong, Azra Yaqub, Naveed K. Janjua, Rizwan Raza, Muhammad Afzal, Bin Zhu, All in One Multifunctional Perovskite Material for Next Generation SOFC, Electrochimica Acta, Volume 193, 1 March 2016, Pages 225-230
8. Chen Xia, Baoyuan Wang, Ying Ma, Yixiao Cai, Muhammad Afzal, Yanyan Liu, Yunjuan He, Wei Zhang, Wenjing Dong, Junjiao Li, Bin Zhu, Industrial-grade rare-earth and perovskite oxide for high-performance electrolyte layer-free fuel cell, Journal of Power Sources, Volume 307, 1 March 2016, Pages 270-279
9. Bin Zhu, Liangdong Fan, Hui Deng, Yunjuan He, Muhammad Afzal, Wenjing Dong, AZRA YAQUB, Naveed K. Janjua, LiNiFe-based layered structure oxide and composite for advanced single layer fuel cells, Journal of Power Sources, Volume 316, 1 June 2016, Pages 37-43
10. Muhammad Afzal, Chen Xia, Bin Zhu, Lanthanum-doped Calcium Manganite (La0.1Ca0.9MnO3) Cathode for Advanced Solid Oxide Fuel Cell (SOFC), Materials Today: Proceedings, Volume 3, Issue 8, 2016, Pages 2698-2706
11. Muhammad Afzal, Mohsin Saleemi, Baoyuan Wang, Chen Xia, Yunjuan He, Jeevan Jayasuriya, Bin Zhu, Fabrication of novel electrolyte-layer free fuel cell with semi-ionic conductor (Ba0.5Sr0.5Co0.8Fe0.2O3-δ- Sm0.2Ce0.8O1.9) and Schottky barrier, Journal of Power Sources, Volume 328, 1 October 2016, Pages 136-142
12. Baoyuan Wang, Yixiao Cai, Chen Xia, Yanyan Liu, Afzal Muhammad, Hao Wang, Bin Zhu, CoFeZrAl-oxide based composite for advanced solid oxide fuel cells, Electrochemistry Communications, Volume 73, December 2016, Pages 15-19
13. Baoyuan Wang, Yixiao Cai, Wenjing Dong, Chen Xia, Wei Zhang, Yanyan Liu, Muhammad Afzal, Hao Wang, Bin Zhu, Photovoltaic properties of LixCo3-xO4/TiO2 heterojunction solar cells with high open-circuit voltage, Solar Energy Materials and Solar Cells, Volume 157, December 2016, Pages 126-133
14. Yuzheng Lua†, Muhammad Afzalb†(equal first author), Bin Zhu*b,c, Baoyuan Wangb,c, Jun Wang*a and Chen Xiab, Nanotechnology Based Green Energy Conversion Devices, Recent Patents on Nanotechnology, 2017, 11, 000-000 (accepted)
15. Chen Xia, Muhammad Afzal, Baoyuan Wang, Aslan Soltaninazarlou, Wei Zhang, Yixiao Cai, Bin Zhu. Mixed-conductive membrane composed of natural hematite and Ni0.8Co0.15Al0.05LiO2-δ for electrolyte layer-free fuel cell. Advanced Materials Letters, 2017, 8(2), 114-121.
16. Chen Xia, Baoyuan Wang, Yixiao Cai, Wei Zhang, Muhammad Afzal, Bin Zhu. Electrochemical properties of LaCePr-oxide/K2WO4 composite electrolyte for low-temperature SOFCs. Electrochemistry Communications, 2016, DOI:10.1016/j.elecom.2016.12.013.
17. Chen Xia, Yixiao Cai, Baoyuan Wang, Muhammad Afzal, Wei Zhang, Aslan Soltaninazarlou, Bin Zhu. Strategy Towards Cost-Effective Low-Temperature Solid Oxide Fuel Cells: A Mixed-conductive Membrane Comprised of Natural Minerals and Perovskite Oxide. Journal of Power Sources, 2017, accepted.​
18. Yanyan Liu, Wei Zhang, Baoyuan Wang, Muhammad Afzal, Chen Xia, Bin Zhu, Industrial Grade LaCe1.85Pr0.03Nd0.06Ox/Na2CO3 Nanocomposite for Novel Low-Temperature Semiconductor-ionic Membrane Fuel Cell. Advanced Materials Letters, 2016, DOI: 10.5185/amlett.2016.1431 (accepted)
Keynote Forum
Katsutoshi Ono
Kyoto University, Japan
Keynote: Batteries and fuel cells are key technologies in the future energy production
Time : 11:10-12:10

Biography:
Katsutoshi Ono received B. Eng. Degree from Kyoto University, Japan, in 1961, degree of Dr. Sci. from Faculté des Sciences, Université de Paris in 1967. He was researcher at Ecole des Mines de Paris, 1965-1967, Professor of Materials Science, Kyoto University, 1982-1997, Energy Science & Technology, 1997-2001. He is Currently Professor Emeritus.
Abstract:
1. Introduction
Concept of “three zeros” power generation system. Zero energy input, Zero matter input, Zero emission.
2. How to achieve” three zeros” power generation system
Hydrogen redox electric power generation, Lithium redox electric power generation.
3. Electrostatic-induction water electrolysis (ESIWE)
Principle, Laboratory experiment details, Direct measurements of electrical power requirements, Nature of the internal energy
creation.
4. Concepts of industrial applications of the three zeros power generation systems
All the constructions of the three zeros power generation systems are assumed to utilize the commercially existing bipolar
water electrolyzers, fuel cells, fuel cell stacks and lithium-ion battery modules, currently operated in industry.
High power application for central station power generation: Hydrogen redox electric power generation system(HREG).,
Combined energy cycle of solar battery, ESI bipolar water electrolyzer and fuel cell.
Low power application for specifi c propulsion systems:
On-board hydrogen redox power generators for infi nite cruising range electric vehicles (Abstract of the Conference). Onboard
lithium redox charge—discharge reciprocating power generator for infi nite cruising range electric vehicles.
5. Conclusive remarks
Th ermodynamic cycle of the three zeros power generation system. Direct electrostatic-to-chemical energy conversion Th ree
zeros power generators are not related to any perpetual motion machine, it works within the laws of thermodynamics through
internal energy creation.
Recent Publications:
1. Ono K: (2015) Energetically self-sustaining electric power generation system based on the combined cycle of electrostatic induction hydrogen electrolyzer and fuel cell IEEJ, Trans. on Fundamentals and Materials, Vol.135 No.1 pp. 22-33.
2. Ono K: (2016) Hydrogen redox electric power and hydrogen energy generators, International Journal of Hydrogen Energy, vol.41 PP.10284-102913.
3. Ono K: (2016) “Hydrogen redox electric power and hydrogen energy generators”, International Journal of Hydrogen Energy, vol.41 P.10284-10291
4. Barbir F, PEM fuel cells, Theory and Prracice, Elsevier, Amsterdam P.268..
5. Annual Report, National Resources and Energies 1999/2000, The Agency for Resources and Energies, Japan.
6. O’ Hayre R, Cha S.W, Collela W and Prinz F.B., (2006) Fuel cell, Fundamentals, John Wiley & Sons INC.
- Primary Cell Batteries| Secondary Cell Batteries| Design and Technology of Batteries| Applications of Batteries| Latest Developments in Batteries
Location: Olimpica 2

Chair
Manfred Martin
RWTH Aachen University, Germany
Session Introduction
Taketoshi Matsumoto
Osaka University, Japan
Title: High capacity anode with Si nanopowder fabricated from swarf for lithium ion battery

Biography:
Taketoshi Matsumoto (Assoc. Prof., Sci, PhD) received his MSc and PhD degrees from Tokyo Institute of Technology, Yokohama, Japan in 2001. From 2000 to 2002, he was a Research Fellow of the Japan Society for the Promotion of Science. Since 2001, he was a Postdoctoral Research Associate in University of Southern California, CA, US. Since 2003, he was a Lecturer in University of Tsukuba, Tsukuba, Japan. Since 2004, he was a Research Associate in Institute for Molecular Science, Okazaki, Japan. Since 2007 and 2014, he has been an Assistant and Associate Professor, respectively, in the Institute of Scientific and Industrial Research, Osaka University, Osaka, Japan. He is interested in energy related nano-materials and devices such as TFTs, LSI, luminous materials, sealed permanent memories, solar cells and Li ion batteries.
Abstract:
Si swarf is generated during slicing Si ingots to produce Si wafers for solar cells. The weight of Si swarf, i.e., industrial waste, is nearly the same as that of Si wafers. Si swarf and Si nanopowder produced by the ball and beads milling methods possesses flake-like shape with length smaller than a few μm and thickness thinner than 40 nm. We have applied Si nanopowder produced from swarf to Li ion batteries, fluorescent materials, a hydrogen generation material, and solar cells. In this study, Si has been applied to high capacity active materials for anodes in Li ion batteries. Si nanoparticles smaller than 150 nm are known to show good cyclability; while for larger Si particles, peeling-off of Si due to their volume change occurs during lithiation and delithiation, resulting in degradation of the cyclability. Si swarf is a promising low-cost material for mass production of Si anodes, while Si nanoparticles have been fabricated so far using high-cost processes such as CVD and laser ablation. Cyclability of a Si anode fabricated from Si swarf is improved by addition of 10-15 wt% fluoroethylene carbonate (FEC) to ethylene carbonate (EC)/diethylene carbonate (DEC)=1/1 electrolyte solutions with 1 M LiPF6. The solutions form a thin and stable solid-electrolyte interphase (SEI) layer, leading to decreases in the SEI resistance (RSEI) and charge transfer resistance (Rct). Carbon-coating (C-coating) on Si also improves the cyclability of the Si anode. Limitation of the delithiation capacity at 1500 mA h/g after deep lithiation at 0.01 V with a Li foil counter electrode also shows better cyclability than that for limitation of the lithiation capacity at 1500 mA h/g after deep delithiation at 1.5 V.
Recent Publications:
1. Matsumoto T, Kimura K, Nishihara H, Kasukabe T, Kyotani T, Kobayashi H (2017) Fabrication of Si nanopowder from Si swarf and application to high-capacity and low cost Li-ion batteries. J. Alloys Compd. 720: 529-540.
2. Kimura K, Matsumoto T, Nishihara H, Kasukabe T, Kyotani T, Kobayashi H (2017) Improvement of cyclability of Li-ion batteries using C-coated Si nanopowder electrode fabricated from Si swarf with limitation of delithiation capacity. J. Electrochem. Soc. 164: A995-A1001.
3. Kasukabe T, Nishihara H, Kimura K, Matsumoto T, Kobayashi H, Kyotani T (2017) Beads-milling of waste Si sawdust into high-performance nanoflakes for lithium-ion batteries. Sci. Rep. 7: 42734-1-10.
4. Matsumoto T, Maeda M, Kobayashi H (2016) Photoluminescence enhancement of adsorbed species on Si. Nanoscale Res. Lett. 11: 7-1-6.
5. Matsumoto T, Maeda M, Furukawa J, Kim W-B, Kobayashi H (2014) Si nanoparticles fabricated from Si swarf by photochemical method. J. Nanopart. Res. 16: 2240-1-7.
6. Maeda M, Imamura K, Matsumoto T, Kobayashi H (2014) Fabrication of Si swarf and application to solar cells. Appl. Surf. Sci. 312: 39-42.
Jun-Hyung Ryu
Dongguk University, South Korea
Title: The development of a decision-making framework for intelligent energy storage system

Biography:
Jun-Hyung Ryu has expertise in Mathematical Programming in the Energy Systems. He has been mainly approached the energy system in the process industry with high energy consumption and concerned with developing the methodology to address multiple energy sources such as renewable energy sources and fossil fuels, and energy storage system. He is an Associate Professor in Department of Nuclear & Energy System Engineering at Dongguk University.
Abstract:
Statement of the Problem: Energy has become an important issue for process industry. In order to survive under tough competition, energy should be secured for the industry in terms of cost and amount. However, continuously varying energy conditions makes it unprecedentedly challenging. Any approach to mitigate the negative impact of the external and internal variation should be developed and implemented. Recently, Energy Storage System (ESS) has been emerging as a competitive tool. There is much to be done to take the full advantage of ESS in practice. This paper aims to highlight the key issues for the practical implementation.
Methodology & Theoretical Orientation: At first, rigorous decision-making framework should be developed for the operation of ESS. Many reports only mention the potential advantages of ESS without describing specific technologies and methodologies. The framework should be closed incorporated with energy system management. The forecasting of supply and demand should be made. The accurate and reliable forecasting allows the ESS to operate in robust manner. Energy and power saving from the perspective of suppliers can be different. The benefit of ESS should be rigorously evaluated by computing the profit. The profit by using ESS should be computed against the ESS set up cost during the entire economic lifespan. ESS is under development. New technologies are constantly developed and tested.
Findings: This presentation highlights the need to urgently develop decision-making frameworks for the systematic operation of ESS. This can be applied to any type of ESS.
Conclusion & Significance: ESS itself cannot generate energy nor consume it. The impact of ESS can be realized when a proper match between suppliers and demands is made. The rising energy and power cost and increasing waste due to the mismatch indicates the importance of ESS in energy systems. More works are expected to take full advantage of ESS.
Recent Publications:
1. Ryu J H, Lee S B, Hodge B M, Lee I B (2016) Techno-economic simulation approach in preparation of employing renewable energies for process industry. IEEE Photovoltaic Specialists Conference 1862-1864
2. Ryu J H, Hodge B M (2016) Mathematical Modelling-based Energy System Operation Strategy considering Energy Storage Systems. Computer Aided Chemical Engineering 38:1455-1460
3. Lee S, Ryu J H, Hodge B M, Lee I B (2016) Development of a Neural Network-based Renewable Energy Forecasting Framework for Process Industries, Computer Aided Chemical Engineering 38: 1527-1532
4. Han J H, Ryu J H, Lee I B(2013), Multi-objective optimization design of hydrogen infrastructures simultaneously considering economic cost, safety and CO2 emission, Chemical Engineering Research and Design 91(8):1427-1439
5. Ryu J H (2013) Developing an integrated capacity planning framework for production processes and demand supply chains, Korean Journal of Chemical Engineering 30: 27-32
Chunman Zheng
National University of Defense Technology, China
Title: Structural Change and Thermal Properties of NCA/LiMn2O4 Blend Materials during the Charging Process

Biography:
Abstract:
Layered oxides LiNi0.8Co0.15Al0.05O2 (NCA) is one of the most attractive cathode material for high power applications such as hybrid/plug-in hybrid electrical vehicles. It shows great benefits in high volumetric energy density and high rate performance. However, there are still some technical hurdles to be solved, such as high temperature stability. The poor high temperature stability is attributed to the chemical reaction of NCA cathode material with the electrolyte in the process of charging and discharging. Blending with other stable cathode materials is one effective way to improve the properties of materials. For example, Tran et al. reported the blend material of LiMn2O4 and NCA, demonstrating very stable cycle performance and high temperature stability. In the present work, NCA/LiMn2O4 blend materials with different ratio were prepared in order to improve the properties of materials. Structural change and thermal properties of NCA/LiMn2O4 blend materials during the charging process were systematically studied. Figure 1 shows the XRD patterns of NCA/LiMn2O4 blend materials which were charged to 4.2 V and 5.0 V, respectively. It shows that the layered cathode materials undergo a reversible phase transition, namely a phase transition from H1 phase to M phase (3.748 V), M phase to H2 phase (4.017 V), and H2 phase to H3 phase (4.227 V). During the phase transition, the cell parameters of blended materials a and b decreases whereas c increases. Figure 2 shows the temperature change of NCA/LiMn2O4 blend materials during the charging process and the high temperature stability of blend materials which were charged to 4.2 V. When the mass ratio of LiMn2O4 is 20%, the blend cathode materials exhibit better anti-overcharge behavior with less heat generated. The decomposition temperature of the blend materials which were charged to 4.2 V is higher than the pure NCA. The blend materials with 20% LiMn2O4 have the best temperature stability.
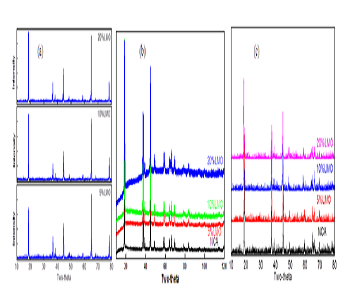
Figure 1: XRD patterns of (a) NCA/LiMn2O4 blend materials, (b) blend materials charged to 4.2V, (c) blend materials charged to 5.0V.
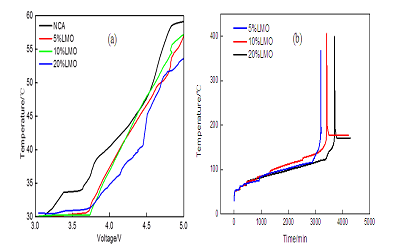
Figure 2: (a) The temperature change of NCA/LiMn2O4 blend materials during the charging process, (b) high temperature stability of blend materials charged to 4.2V.
Recent Publications:
1. Li X, Xie Z, Liu W, Ge W, Wang H, and Qu M. Effects of fluorine doping on structure, surface chemistry, and electrochemical performance of LiNi0.8Co0.15Al0.05O2 [J]. Electrochimica Acta, 2015, 174: 1122-1130.
2. Haselrieder W, Ivanov S, Tran H Y, Theil S, Froböse L. Influence of Formulation Method and Related Processes on Structural, Electrical and Electrochemical properties of LMS/NCA-blend Electrodes [J]. Progress in Solid State Chemistry, 2014, 42(4): 157-174.
3. Tran H Y, Täubert C, Fleischhammer M, Axmann P, Küppers L and Mehrens M W. LiMn2O4 Spinel/ LiNi0.8Co0.15Al0.05O2 Blends as Cathode Materials for Lithium-ion batteries [J]. Journal of the Electrochemical Society, 2011, 158(5): A556-A561.
Katsutoshi Ono
Kyoto University, Japan
Title: Theories of on-board hydrogen redox electric power generators for infinite cruising range electric vehicles

Biography:
Katsutoshi Ono received B. Eng. Degree from Kyoto University, Japan, in 1961, degree of Dr. Sci. from Faculté des Sciences, Université de Paris in 1967. He was researcher at Ecole des Mines de Paris, 1965-1967, Professor of Materials Science, Kyoto University, 1982-1997, Energy Science & Technology, 1997-2001. He is Currently Professor Emeritus.
Abstract:
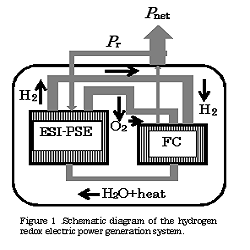
This paper presents the theoretical aspects of a specific propulsion system for electric vehicles, based on a prototype standard passenger fuel cell vehicle (FCV), termed a hydrogen redox electric power generator (HREG). This generator utilizes a combined energy cycle composed of a fuel cell that produces power and an electrostatic- induction potential superposed water electrolytic cell (ESI-PSE) for the synthesis of a stoichiometric H2/O2 fuel for the fuel cell. To allow an essentially infinite cruising range, the fuel must be synthesized while the car is in motion. In this scenario, the HREG offers considerable advantages. Firstly, this device is capable of functioning with zero matter and energy inputs and generates no emissions, without violating the laws of thermodynamics. The H2O → H2 +1/2O2 reduction reaction proceeds in the ESI-PSE, which functions on a so-called “zero power input” mechanism involving the conversion of electrostatic energy to chemical energy. In this unit, the power used is 17% of the total electrical energy that is theoretically required, while the remaining 83% can be provided by electrostatic energy free of power. Part of the power delivered by the fuel cell is returned to the ESI-PSE cell, while the remainder represents the net power output used to drive the electric motor. A second advantage is that the HREG system can be employed on both a large scale, such as in the case of a central power station, and on a much smaller scale, such as for on-board electric power generation in an electric vehicle. The present work performed a theoretical assessment of this new propulsion system for electric vehicles, focusing on the following three aspects:
1. the power electronics circuit connected to the ESI-PSE cell,
2. the performance of the on-board HREG system,
3. the lithium-ion battery charge-discharge reciprocating electric power generator.
Recent Publications:
1. Ono K: (2015) Energetically self-sustaining electric power generation system based on the combined cycle of electrostatic induction hydrogen electrolyzer and fuel cell IEEJ, Trans. on Fundamentals and Materials, Vol.135 No.1 pp. 22-33.
2. Ono K: (2016) Hydrogen redox electric power and hydrogen energy generators, International Journal of Hydrogen Energy, vol.41 PP.10284-102913.
3. Ono K: (2016) “Hydrogen redox electric power and hydrogen energy generators”, International Journal of Hydrogen Energy, vol.41 P.10284-10291
4. Barbir F, PEM fuel cells, Theory and Prracice, Elsevier, Amsterdam P.268..
5. Annual Report, National Resources and Energies 1999/2000, The Agency for Resources and Energies, Japan.
6. O’ Hayre R, Cha S.W, Collela W and Prinz F.B., (2006) Fuel cell, Fundamentals, John Wiley & Sons INC.
Shridhar Pandey
Ramway New Energy Co. Ltd., China
Title: Primary lithium batteries passivation characteristics and effects in LiSOCl2 chemistries
Time : 14:50-15:15

Biography:
Shridhar Pandey is working as a Country Head at Ramway New Energy Co. Ltd., China. He has completed his graduation in Electronics and Communications Engineering at Visvesvaraya Technological University, Belgaum. Being an adaptable and innovative Engineer with over three years of experience in Manufacturing and Marketing, he has excellent team leading capabilities. He has been working since a year and focusing on Lithium Battery Technology for AMI/AMR, Smart Meters and Energy Meters. He has introduced Ramway batteries to utility companies and Indian market. He has also been successful in writing articles and research papers based on batteries and AMI for various Indian magazines and journals.
Abstract:
Passivation is a phenomenon for lithium battery; a thin chemical film appears on the surface of lithium anode and prevents it from oxidation which is forming on the surface of the metal. In lithium-thionyl chloride battery, thionyl chloride is a liquid. Lithium anode gets in touch with thionyl chloride and the oxidation reaction will start slowly. The outcome of this oxidation is lithium chloride. The lithium chloride which is formed on the surface of the lithium anode is very small and it prevents the chemical reaction between lithium and thionyl chloride. This phenomenon of lithium is called as passivation. The passivation in lithium thionyl chloride batteries starts as soon as the batteries are manufactured, but the reaction is not fast. The passivation is directly proportional to the temperature. With the increase in temperature, the rate of passivation increases. The passivation is more dangerous as longer the time is. Passivation is the intrinsic characteristics of lithium thionyl chloride battery. It is impossible to store lithium thionyl chloride batteries without passivation. The lithium chloride is formed on the surface of the lithium anode in thionyl chloride is very dense which prevents the reaction between lithium and thionyl chloride. Because of passivation the self-discharge rate inside the cell becomes very small. By this way, we can achieve the shelf life of 10 years for lithium thionyl chloride batteries. This is the positive sign of passivation. Passivation protects the battery capacity and prevents it from capacity loss. In this presentation, we are going to deliver how to save batteries from getting passivized and how it can be depassivized.
Recent Publications:
1. Shridhar Pandey, Anish Garg(2017) Two Pole Latching relays for Smart Electricity Meters at Metering India 2017
2. Shridhar pandey, Anish Garg(2016) Performance, testing and Reliability of Two Pole Latching relays for smart Energy meters at World energy Congress 2016, Italy
3. Shridhar pandey, Anish Garg(2016) Performance, testing and Reliability of Two Pole Latching relays for smart Energy meters Renewable energy week, London UK 2016
4. Shridhar pandey, Anish Garg(2016) Performance, testing and Reliability of Two Pole Latching relays for smart Energy meters at Water and Energy International Journal, New Delhi
Yujie Li
National University of Defense Technology, China
Title: Nano-sized lithium-rich cathode 0.4Li2 MnO3 0.6Li[Ni1/3Mn1/3Co1/3]O2 prepared by resorcinol-formaldehyde assisted sol-gel method

Biography:
Yujie Li studies energy materials and their electrochemistry, including synthesis of cathode material, anode material, and all-solid lithium battery as well as their applications in lithium storage and conversion. He received his Bachelor’s degree in Materials Chemistry at Lanzhou University, China in 2003; Master’s degree in Materials Physics Chemistry in 2006 and; Doctor’s degree in Materials Science & Engineering in 2010 at National University of Defense Technology, China. He is now an Assistant Professor in Department of Materials Science & Engineering at National University of Defense Technology, China.
Abstract:
Recently, layered lithium-rich cathode materials, xLi2MnO3 (1-x)LiMO2(M=transition metal) have been considered as one of the most promising cathode materials due to their high specific capacity (200-300 mAhg-1) and high operating voltage. However, lithium-rich cathode materials still suffer from several major defects, such as low coulomb efficiency, the poor long-term capacity retention, and the inferior rate performance. As we know, nano-sized cathode is an effective method, the nano dimension provided more active surface sites for lithium storage, and also shortened the lithium-diffusion pathways, and these could improve electrochemistry performance. Herein, we prepared nano particle lithium-rich layered 0.4Li2MnO30.6Li[Ni1/3Mn1/3Co1/3]O2 by resorcinol-formaldehyde assisted sol-gel method. Nano-sized lithium-rich cathode presented excellent cycling performance. It showed a high initial discharge capacity of 256.8mAh g-1 and an initial coulomb efficiency of 78.9%. The discharge capacity after 50 cycles was 240.2mAh g-1 with capacity retention of 93.5% due to more active surface sites and shortens lithium-diffusion pathways of nanoparticles. Figure 1(a)(b) demonstrate its SEM images. Lithium-rich nanoparticles are about 250 nm-300 nm. Figure 1(c) showed the XRD pattern, it formed the good layered crystallizability. Figure 1(d) demonstrates, it has good capacity retention. These results indicate the nano-sized lithium-rich cathode could effectively suppress capacity attenuation and enhance coulomb efficiency and cycling performance.
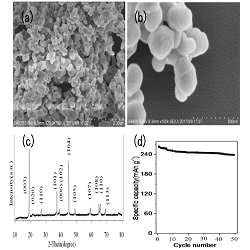
Figure 1: (a) (b) SEM, (c) XRD and (d) cycling performance (0.1C rate) of 0.4Li2MnO3 0.6Li[Ni1/3Mn1/3Co1/3]O2 nanoparticles
Recent Publications:
1. Wang C-C, Jarvis K A, Ferreira P J, Manthiram A. Effect of Synthesis Conditions on the First Charge and Reversible Capacities of Lithium-Rich Layered Oxide Cathodes[J]. Chem. Mater., 2013, 25(15): 3267-3275.
2. Pramanik A, Ghanty C, Majumder S B. Synthesis and electrochemical characterization of xLi(Ni0.8Co0.15Mg0.05)O2· (1-x)Li[Li1/3Mn2/3]O2(0.0
Liu Shuangke
National University of Defense Technology, China
Title: Graphene oxide coated S particles with long cycle life for lithium sulfur battery

Biography:
Liu Shuangke studies energy materials and electrochemistry, including synthesis of metal oxides, nano-carbons, sulfur-carbon composites as well as their applications in lithium storage and conversion. He received his Bachelor’s degree in Materials Science & Engineering at Hunan University, China in 2010; Master’s degree in Applied Chemistry in 2012 and; Doctorate degree in Materials Science & Engineering in 2016 at National University of Defense Technology, China. He is now an Assistant Professor in Department of Materials Science & Engineering at National University of Defense Technology, China.
Abstract:
Lithium sulfur battery has been regarded as one of the most promising high energy density rechargeable energy storage system for next generation due to its high theoretical specific capacity/energy density, natural abundance and environmental friendliness. However, the rapid capacity fade during long cycles which is caused by polysulfide shuttle and volume expansion during cycles tremendously inhibits its practical application. Building coated architecture of sulfur is one effective way to confine sulfur in the sulfur cathode thus enables stable cycle life. For example, Zhou et al. reported a sulfur/GO core-shell particle, in which sulfur particle were well wrapped by GO, demonstrating very stable cycle performance up to 1000 cycles. Herein, we also prepared GO-coated sulfur particles to enhance the cycle performance of lithium sulfur battery. Different from the above example which uses milled nano-sulfur particles, we use sodium sulfide and sodium sulfite to generate sulfur particles in aqueous GO solution followed by centrifugation and freeze-drying process, which results in in situ coating structure. The SEM (Fig. 1 a) and TEM (Fig. 1 b, c) images clearly demonstrate sulfur particles are well wrapped by wrinkled GO sheets, the high resolution TEM result indicate the crystal phase of sulfur particles. Due to the intact coating structure of GO and good chemical bond between sulfur and GO, the GO-coated sulfur particle cathode shows excellent cycling performance. Figure 1d demonstrates its cycle performance at 1 C rate, the initial discharge capacity is 513.4 mAhg-1, with 467.4 mAhg-1 retained after 400 cycles, corresponding to capacity retention of 91%. However, after 400 cycles, the capacity fades starts to speed up with 260.6 mAhg-1 and 225.1 mAhg-1 left after 800 and 1000 cycles. These results indicate that the physical GO coating could effectively suppress polysulfides shuttle effect and enhance the cycling performance but cannot completely eliminate it.
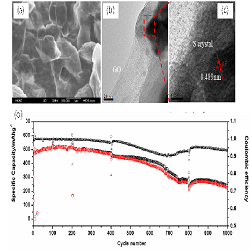
Figure 1: (a) SEM, (b) TEM, (c) high resolution TEM and (d) cycling performance at 1C rate of GO-coated S particles
Recent Publications:
1. Manthiram A, Fu Y, Chung S.H, Zu C, Su Y.S. Rechargeable Lithium-Sulfur Batteries, Chem. Rev. 2014, 114(23):11751-11787.
2. Wang D W, Zeng Q, Zhou G, Yin L, Li F, Cheng H M, Gentle I, Lu G. Carbon-sulfur composites for Li-S batteries: status and prospects. J. Mater. Chem. A, 2013, 1 (33): 9382~9394.
3. Rong J.P, Ge M.Y, Fang X, Zhou C.W. Solution Ionic Strength Engineering as a Generic Strategy to Coat Graphene Oxide (GO) on Various Functional Particles and Its Application in High-Performance Lithium-Sulfur (Li-S) Batteries, Nano Lett., 2014, 14,473-479.
Jungmyoung Kim
Changwon national University, South Korea
Title: An analysis of vanadium redox flow battery performance under various operating conditions

Biography:
Jungmyoung Kim earned his Master’s degree in Vanadium Redox Flow Battery Studies from Changwon National University in 2017. He is currently a PhD student at the Nano thermofluidics Energy Transfer Lab of Mechanical Engineering Department of Changwon National University in South Korea.
Abstract:
The vanadium redox flow battery (V-RFB) used in a large-capacity energy storage system is a semi-permanent secondary battery having a relatively long life span and low electrolyte contamination due to cross-over. The performance of the V-RFB system depends on conditions such as electrolyte flow rate and temperature, which are key operating variables. This paper devises four reservoir systems with a single cell 25 cm2 reaction areas and temperature, flow rate control to understand the general thermal and electrochemical reaction characteristics of V-RFB. Also, experimental analysis of a single cell is presented by measuring polarization curves according to experimental variables. The polarization curves are measured in the low current density region without the influence of the concentration overpotential, and the electrolyte temperatures are 278 K, 298 K, and 318 K. the current density of the constant current discharge from 50 A/m2 to 300 A/m2 and the flow rates are 20 mL/min, 60 mL/min, and 100 mL/min. In the polarization curve analysis, the influence of the activation overpotential according to the experimental conditions is applied to the Tafel theory, and the influence of the concentration loss is ignored. The electron transfer coefficient increases as the electrolyte temperature and flow rate increase, while the exchange current density is obtained as described in the Arrhenius equation. The overpotential due to the resistance is represented by the area specific resistance and decreases to the ration of 20.3 (mΩ cm2)/K as the temperature of the electrolyte increases. In the analysis, we successfully found the electrochemical parameters and resistances of the overpotential by verifying various temperature and flow rate.
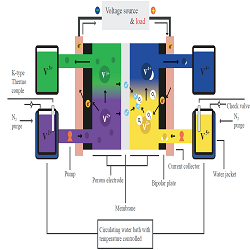
Figure 1: Single cell schematic with temperature control system and four electrolyte reservoirs.
Recent Publications:
1. Ulaganathan M, et al. (2016) Recent advancements in all-vanadium redox flow batteries. Advanced materials interfaces 3.1.
2. Rose M A, Williamson M A, Willit J (2015) Determining the exchange current density and Tafel constant for uranium in LiCl/KCl eutectic. ECS electrochemistry letters 4.1:C5-C7.
3. Xiao S, et al. (2016) Broad temperature adaptability of vanadium redox flow battery—Part 1: Electrolyte research. Electrochimica acta 187:525-534.
4. Zhang C, et al. (2015) Effects of operating temperature on the performance of vanadium redox flow batteries. Applied energy 155:349-353.
5. Mohamed M R, Leung P K, Sulaiman M H (2015) Performance characterization of a vanadium redox flow battery at different operating parameters under a standardized test-bed system. Applied energy 137:402-412
Ashley Brew
OXIS Energy Ltd, UK
Title: Microgrid energy storage using Lithium-Sulfur Batteries: Feasibility of solvent-in-salt electrolytes

Biography:
Ashley Brew gained his MSc in Catalysis and PhD in Electrocatalysis from Cardiff University, specializing in the oxygen reduction reaction in fuel cells. After a few post-doctoral positions in the fields of EPR spectroscopy and thermoelectric materials, he now works at OXIS Energy, a leading developer of lithium-sulfur battery chemistry. As a research scientist at OXIS, Ashley is a member of the electrolyte research group and is currently nearing the end of a 12-month project that aims to establish the feasibility of novel electrolytes for microgrid applications. OXIS Energy also develop lithium-sulfur secondary batteries for several other applications, including automotive and aerospace.
Abstract:
Lithium-Sulfur (Li-S) batteries are considered one of the most promising technologies that could provide a generational leap in terms of energy density over current lithium ion batteries. OXIS Energy have demonstrated this by succeeding in developing Li-S batteries at 400Wh/kg. However, capacity fade at such high energy density is rapid and further research and development is needed to alleviate this. Many factors contribute to capacity fade in Li-S batteries: for example, dissolution and loss of cathode material, consumption of the electrolyte due to its reaction with lithium metal and electrical isolation of insulating sulfur and Li2S charge and discharge products. Another major issue in Li-S batteries is the ‘polysulfide shuttle’, in which reaction intermediates shuttle between the cathode and anode during charge. So-called solvent-in-salt (SIS) electrolytes are those in which the salt exceeds the solvent either by weight, by volume, or both. These unique electrolytes have demonstrated interesting properties in the literature and may solve many of the problems outlined above. SIS electrolytes inhibit intermediate dissolution due to the common ion effect, thus reducing active material loss and inhibiting the polysulfide shuttle. SIS electrolytes have also demonstrated improved lithium plating due to the high lithium-ion transference number, leading to lower rates of electrolyte depletion. These combinations of factors have resulted in these electrolytes exhibiting excellent cycle stability and coulombic efficiency in literature studies. Here we will present our work developing this type of electrolyte for R&D pouch cells and their possible use in microgrid energy storage applications.
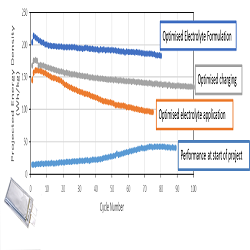
Recent Publications:
1. M. Wild, L. O'Neill, T. Zhang, R. Purkayastha, G. Minton, M. Marinescub and G. J. Offer, Energy Environ. Sci., 2015, 8, 3477-3494.
2. T. Zhang, M. Marinescu, L. O'Neill, M. Wild and G. Offer Phys. Chem. Chem. Phys., 2015, 17, 22581-22586.
3. A. Fotouh, D. J. Auger, K. Propp, S. Longo, M. Wild, Renewable and Sustainable Energy Reviews, 2016, 56, 1008-1021.
4. K. Propp, M. Marinescu, D. J. Auger, L. O'Neill, A. Fotouh, K. Somasundaram, G. Offer, G. Minton, Journal of Power Sources, 2016, 328, 289-299
5. G.Minton, L. Lue, Journal of Molecular Physics, 2016, 114, 16-17, 2477-2491









