
Jungmyoung Kim
Changwon national University, South Korea
Title: An analysis of vanadium redox flow battery performance under various operating conditions
Biography
Biography: Jungmyoung Kim
Abstract
The vanadium redox flow battery (V-RFB) used in a large-capacity energy storage system is a semi-permanent secondary battery having a relatively long life span and low electrolyte contamination due to cross-over. The performance of the V-RFB system depends on conditions such as electrolyte flow rate and temperature, which are key operating variables. This paper devises four reservoir systems with a single cell 25 cm2 reaction areas and temperature, flow rate control to understand the general thermal and electrochemical reaction characteristics of V-RFB. Also, experimental analysis of a single cell is presented by measuring polarization curves according to experimental variables. The polarization curves are measured in the low current density region without the influence of the concentration overpotential, and the electrolyte temperatures are 278 K, 298 K, and 318 K. the current density of the constant current discharge from 50 A/m2 to 300 A/m2 and the flow rates are 20 mL/min, 60 mL/min, and 100 mL/min. In the polarization curve analysis, the influence of the activation overpotential according to the experimental conditions is applied to the Tafel theory, and the influence of the concentration loss is ignored. The electron transfer coefficient increases as the electrolyte temperature and flow rate increase, while the exchange current density is obtained as described in the Arrhenius equation. The overpotential due to the resistance is represented by the area specific resistance and decreases to the ration of 20.3 (mΩ cm2)/K as the temperature of the electrolyte increases. In the analysis, we successfully found the electrochemical parameters and resistances of the overpotential by verifying various temperature and flow rate.
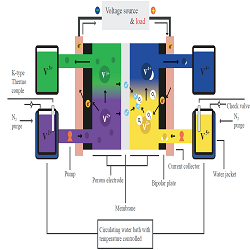
Figure 1: Single cell schematic with temperature control system and four electrolyte reservoirs.
Recent Publications:
1. Ulaganathan M, et al. (2016) Recent advancements in all-vanadium redox flow batteries. Advanced materials interfaces 3.1.
2. Rose M A, Williamson M A, Willit J (2015) Determining the exchange current density and Tafel constant for uranium in LiCl/KCl eutectic. ECS electrochemistry letters 4.1:C5-C7.
3. Xiao S, et al. (2016) Broad temperature adaptability of vanadium redox flow battery—Part 1: Electrolyte research. Electrochimica acta 187:525-534.
4. Zhang C, et al. (2015) Effects of operating temperature on the performance of vanadium redox flow batteries. Applied energy 155:349-353.
5. Mohamed M R, Leung P K, Sulaiman M H (2015) Performance characterization of a vanadium redox flow battery at different operating parameters under a standardized test-bed system. Applied energy 137:402-412

